Abstract
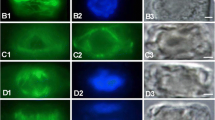
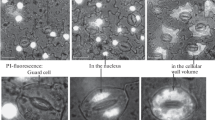
Protoplasts were prepared from the guard cells ofA. cepa. Epidermal peels taken from expanding green leaves and largely free of mesophyll were treated with Cellulysin, and protoplasts were harvested after 18 h of digestion. That the protoplasts were derived from guard cells was ascertained from their characteristic vacuolar autofluorescence and from observations showing that all other epidermal cells are killed in the peeling procedure. The protoplasts proved to be a good system with which to view the cell cortex and inner surface of the plasmalemma. The lysis of cells adhering to polylysine-treated, Formvar-coated grids, followed by negative staining in uranyl acetate, showed that many microtubules normally present in ordered arrays in situ remain closely applied to the inner surface of the plasmalemma in protoplasts. In addition, numerous vesiculate elements including coated vesicles and/or pits are present amongst the microtubules. Similar vesicles are evident in thin sections of fixed, embedded guard cells and protoplasts. The significance of these structures in the cell cortex is discussed.
Similar content being viewed by others
References
Albertini, D.F., Clark, J.I. (1975) Membrane-microtubule interactions: concanavalin A capping induced redistribution of cytoplasmic microtubules and colchicine binding proteins. Proc. Natl. Acad. Sci. USA72, 4976–4980
Allen, N.S., Allen, R.D. (1978) Cytoplasmic streaming in green plants. Annu. Rev. Biophys. Bioeng.7, 497–526
Anderson, R.G.W., Vasile, E., Mello, R.J., Brown, M.S., Goldstein, J.L. (1978) Immunocytochemical visualization of coated pits and vesicles in human fibroblasts: relation to low density lipoprotein receptor distribution. Cell15, 919–933
Behnke, O. (1975) An outer component of microtubules. Nature (London)257, 709–710
Bloodgood, R.A., Leffler, E.M., Bojczuk, A.T. (1979) Reversible inhibition ofChlamydomonas surface motility. J. Cell Biol.82, 664–674
Brown, M.S., Goldstein, J.L. (1979) Receptor-mediated endocytosis: insights from the lipoprotein receptor system. Proc. Natl. Acad. Sci. USA76, 3330–3337
Clarkson, D.T. (1977) Membrane structure and transport. In: Molecular biology of plant cells, pp. 24–63, Smith, H., ed. University of California Press, Berkeley California
Cram, W.J. (1980) Pinocytosis in plants. New Phytologist84, 1–17
Franke, W.W., Herth, W. (1974) Morphological evidence for de novo formation of coated vesicles in exponentially growing cultured plant cells. Exp. Cell. Res.89, 447–451
Franke, W.W., Luder, M.R., Kartenbeck, J., Zerban, H., Keenan, T.W. (1976) Involvement of vesicle coat material in casein secretion and surface regeneration. J. Cell Biol.69, 173–195
Giddings, T.H., Brower, D.L., Staehelin, L.A. (1980) Visualization of particle complexes in the plasma membrane ofMicrasterias denticulata associated with the formation of cellulose fibrils in primary and secondary cell walls. J. Cell Biol84, 327–339
Goldstein, J.L., Anderson, R.G.W., Brown, M.S. (1979) Coated pits, coated vesicles and receptor-mediated endocytosis. Nature (London)279, 679–685
Hardham, A.R., Gunning, B.E.S. (1978) Structure of cortical microtubule arrays in plant cells. J. Cell Biol.77, 14–34
Hardham, A.R., Gunning, B.E.S. (1979) Interpolation of microtubules into cortical assays during cell elongation and differentiation in the roots ofAzolla pinnata. J. Cell Sci.37, 411–442
Heath, I.B. (1974) Unified hypothesis for role of membrane-bound enzyme complexes and microtubules in plant-cell wall synthesis. J. Theor. Biol.48, 445–449
Helenius, A., Kartenbeck, J., Simons, K., Fries, E. (1980) On the entry of Semliki Forest virus into BHK-21 cells. J. Cell Biol.84, 404–420
Hepler, P.K., Palevitz, B.A. (2974) Microtubules and microfilaments. Annu. Rev. Plant Physiol.25, 309–362
Heuser, J. (1980) Three-dimensional visualization of coated vesicle formation in fibroblasts. J. Cell Biol.84, 560–583
Jacobs, M., Bennett, P.M., Dickens, M.J. (1975) Duplex microtubule is a new form of tubulin assembly induced by polycations. Nature (London)257, 707–709
Keen, J.H., Willingham, M.C., Pastan, I.H. (1979) Clathrin-coated vesicles: isolation, dissociation and factor-dependent reassociation of clathrin baskets. Cell16, 303–312
Kirschner, M.W., Honig, L.S., Williams, R.C. (1975) Quantitative electron microscopy of microtubule assembly in vitro. J. Mol. Biol.99, 263–276
Lloyd, C.W., Slabas, A.R., Powell, A.J., Lowe, S.B. (1980) Microtubules, protoplasts and cell shape. Planta140, 7–14
Marchant, H.J., Hines, E.R. (1979) The role of microtubules and cell-wall deposition in elongation of regenerating protoplasts ofMougeotia. Planta146, 41–48
Maxfield, F.R., Willingham, M.C., Schlessinger, J., Davies, P.J.A., Pastan, I. (1979) Receptor-mediated internalization of proteins and polypeptide hormones by cultured fibroblasts. In: Hormones and cell culture. Cold Spring Harbor conferences on cell proliferation, vol. 6, pp. 159–166. Cold Spring Harbor Press, Cold Spring Harbor, N.Y. USA
Mazia, D., Mazia, G., Sale, Schatten and Sale, W. (1975) Adhesion of cells to surfaces coated with polylysine. J. Cell Biol.66, 198–200
Newcomb, E.H. (1980) Coated vesicles: their occurrence in different plant cell types. In: Coated vesicles, pp. 55–68, Ockleford, C.D., Whyte, A., eds., Cambridge University Press, Cambridge, U.K.
Nicolson, G.L. (1976) Cytoplasmic influence over cell surface components. Biophys. Biochim. Acta457, 57–108
Olmsted, J.B., Marcum, J.M., Johnson, K.A., Allen, C., Borisy, G.G. (1974) Microtubule assembly: some possible regulatory mechanisms. J. Supramol. Struct.2, 429–450
Palevitz, B.A. (1976) Actin cables and cytoplasmic streaming in green plants. In: Cell motility, Bk.C, pp. 601–611, Goldman, R., Pollard, T., Rosenbaum, J., eds. Cold Spring Harbor Press, Cold Spring Harbor, N.Y., USA
Palevitz, B.A. (1980a) Comparative effects of phalloidin and cytochalasin B on motility and morphogenesis inAllium. Can. J. Bot.58, 773–785
Palevitz, B.A. (1980b) The structure and development of stomatal cells. In: Soc. Exp. Biol. Sem. Ser., Stomatal Physiology, Jarvis, P.G., Mansfield, T.A., eds. Cambridge University Press, Cambridge, U.K., in press
Palevitz, B.A., Hepler, P.K. (1976) Cellulose microfibril orientation and cell shaping in developing guard cells ofAllium—role of microtubules and ion accumulation. Planta132, 71–93
Pearse, B.M.F. (1976) Clathrin: a unique protein associated with intracellular transfer of membrane by coated vesicles. Proc. Natl. Acad. Sci. USA73, 1255–1259
Reynolds, E.S. (1963) The use of lead citrate at high pH as an electron opaque stain in electron microscopy. J. Cell Biol.17, 208–212
Robinson, D.G. (1977) Plant cell wall synthesis. Adv. Bot. Res.5, 89–151
Roth, T.F., Porter, K.R. (1964) Yolk protein uptake in the oocyte of the mosquitoAedes aegypti L. J. Cell Biol.20, 313–332
Rothman, J.E., Fine, R.E. (1980) Coated vesicles transport newly synthesized membrane glycoproteins from endoplasmic reticulum to plasma membrane in two successive stages. Proc. Natl. Acad. Sci. USA77, 780–784
Schnabl, H., Bornman, C.H., Ziegler, H. (1978) Studies on isolated starch-containing (Vicia faba) and starch-deficient (Allium cepa) guard cell protoplasts. Planta143, 33–39
Seagull, R.W., Heath, I.B. (1979) The effects of tannic acid on the in vivo preservation of microfilaments. Eur. J. Cell Biol.20, 184–188
Singh, A.P., Srivastava, L.M. (1973) The fine structure of pea stomata. Protoplasma76, 61–82
Spurr, A.R. (1969) A low-viscosity epoxy resin embedding medium for electron microscopy. J. Ultrastruct. Res.26, 31–43
Srivastava, L., Singh, A.P. (1972). Stomatal structure in corn leaves. J. Ultrastruct. Res.39, 345–363.
Willingham, M.C., Maxfield, F.R., Pastan, I.H. (1979) 401-1 Macroglobulin binding to the plasma membrane of cultured fibroblasts. Diffuse binding followed by clustering in coated pits. J. Cell Biol.82, 614–625
Woodward, M.P., Roth, T.F. (1978) Coated vesicles: characterization, selective dissociation and reassembly. Proc. Natl. Acad. Sci. USA75, 4394–4398
Zeiger, E., Hepler, P.K. (1976) Production of guard cell protoplasts from onion and tobacco. Plant. Physiol.58, 492–498
Zeiger, E., Hepler, P.K. (1977) Light and stomatal function: blue light stimulates swelling of guard cell protoplasts. Science196, 887–889
Zeiger, E., Hepler, P.K. (1979) Blue light-induced, intrinsic vacuolar fluorescence in onion guard cells. J. Cell Sci.37, 1–10
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Doohan, M.E., Palevitz, B.A. Microtubules and coated vesicles in guard-cell protoplasts ofAllium cepa L.. Planta 149, 389–401 (1980). https://doi.org/10.1007/BF00571175
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00571175