Summary
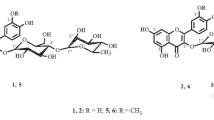
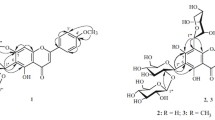
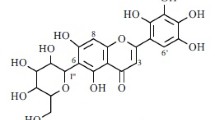
New flavonoid glycosides have been obtained from the roots ofRhodiola algida: rhodalgin (I), composition C20H18O11, mp 239–240°C; acetylrhodalgin (II) C22H20O12, mp 223–224°C; diacetylrhodalgin (III), C24H22O13, mp 208–209°C; and triacetylrhodalgin (IV), C26H24O14, mp 230–231°C.
It has been established that they have the following structures: (I), 3,4′,5,7,8-pentahydroxyflavone 8-O-α-L-arabinopyranoside; (II), 3,4′,5,7,8-pentahydroxyflavone 8-O-(3″-O-acetyl-α-L-arabinopyranoside; (III), 3,4′,5,7,8-pentahydroxyflavone 8-O-(2″,3″-di-O-acetyl)-β-D-xylopyranoside; and (IV), 3,4′,5,7,8-pentahydroxyflavone 8-O-(2″,3″4″-tri-O-acetyl)-β-D-xylopyranoside. The α-L-arabinopyranose and β-D-xylopyranose are present in these compounds in the C1 conformations.
In the performance of this investigation, the authors consulted O. S. Chizhov, and M. B. Zoltarev (N. D. Zelinskii Institute of Organic Chemistry of the Academy of Sciences of the USSR) and V. I. Sheichenko (All-Union Institute of Medicinal plants).
Similar content being viewed by others
Literature cited
T. T. Pangarova, G. G. Zapesochnaya, and E. L. Nukhimovskii, “The flavonoids of Rhodiola algida,” Khim. Prirodn. Soedin., 667 (1974).
G. G. Zapesochnaya and T. T. Pangarova, Khim. Prirodn. Soedin., 554 (1973).
T. J. Mabry, K. R. Markham, and M. B. Thomas, The Systematic Identification of Flavonoids, Springer, New York (1970), p. 125.
E. Rodriguez, N. J. Carman, and T. J. Mabry, Phytochem.,11, 409 (1972).
R. G. Wilson, J. H. Bowie, and D. H. Williams, Tetrahedron,24, 1407 (1966).
J. H. Bowie and D. W. Cameron, Aust. J. Chem.,19, 1627 (1966).
D. G. I. Kingston, Tetrahedron,27, 2691 (1971).
N. K. Kochetkov and O. S. Chizhov, Advan. Carbohydr. Chem.,21, 39 (1966).
H. Budzikiewicz, C. Djerassi, and D. H. Williams, Structure Elucidation of Natural Products by Mass Spectrometry, Holden-Day, San Francisco, Vol. 2 (1964), p. 203.
C. V. Holland, D. Horton, M. J. Miller, and N. S. Bhacca, J. Org. Chem.,32, 3077 (1967).
H. Paulsen and F. Leupold, Carbohydr. Res.,3, 47 (1966).
L. D. Hall, Chem. Ind. (London), 950 (1963).
R. J. Abraham, L. D. Hall, L. Hough, and K. A. McLauchlan, J. Chem. Soc., 3699 (1962).
R. U. Lemieux, Can. J. Chem.,39, 116 (1961).
Additional information
All-Union Scientific-Research Institute of Medicinal Plants. Translated from Khimiya Prirodnykh Soedinenii, No. 6, pp. 712–720. November–December, 1975.
Rights and permissions
About this article
Cite this article
Pangarova, T.T., Zapesochnaya, G.G. The structure of the flavonoids from Rhodiola algida. II. Chem Nat Compd 11, 744–750 (1975). https://doi.org/10.1007/BF00568460
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF00568460