Abstract
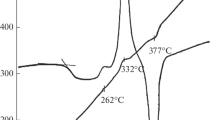
By the deliberate addition of oxygen (up to 1.0 at. % in the form of GeO2), its effect on the structure and properties of quenched Ge-Te glasses was studied. The glass-forming region of the water-quenched melts is narrowed by the addition of oxygen impurity. Replica electron micrographs of the glasses show apparent coarsening of the microstructure by such an oxygen addition, whereas the crystallization behaviour of the splat-cooled melts studied by DTA did not change significantly with this oxygen addition. Morphological features of GeTe crystallites in the glass + crystal portion observed in the oxygen-containing melts are described. However, in cases when either the melts were splat-cooled in an oxidizing atmosphere, or the DTA runs were made in air, a two-stage crystallization reaction was observed on DTA thermograms. Reactivity of the melts with SiO2 glass vials is enhanced by the existence of the impurity oxygen.
Similar content being viewed by others
References
G. Hetherington and K. H. Jack, Phys. Chem. Glasses 3 (1962) 129.
T. Bell, G. Hetherington, and K. H. Jack, ibid 3 (1962) 141.
R. W. Lee, ibid 5 (1964) 35.
J. A. Savage and S. Nielsen, ibid 5 (1964) 82.
Idem, ibid 6 (1965) 90.
Idem, ibid 7 (1966) 56.
Idem, Infrared Phys. 5 (1965) 195.
J. A. Muir and R. J. Cashman, J. Opt. Soc. Amer. 57 (1967) 1.
A. R. Hilton and C. E. Jones, Phys. Chem. Glasses 7 (1966) 112.
A. R. Hilton, Appl. Opt. 5 (1966) 1877.
J. Non-Cryst. Solids, 2 and 4 (1970).
W. C. Lacourse, V. A. Twaddell, and J. D. Mackenzie, ibid 3 (1970) 234.
Takeshitakamori, Rustum Roy, and Gregory J. McCarthy, Mat. Res. Bull. 5 (1970) 529.
E. R. Plumat, J. Amer. Ceram. Soc. 51 (1968) 499.
Yoji Kawamoto and Shoji Tsuchihashi, ibid 52 (1969) 626.
J. A. Savage, J. Mater. Sci. 6 (1971) 964.
H. Krebs and P. Fischer, Presented at the Faraday Society Discussion Meeting, Bristol, September 22–24, 1970.
Sigeruiizima, Michiosugi, Makoto Kikuchi, and K. Tanaka, Solid State Communications 8 (1970) 153.
A. R. Hilton, C. E. Jones, and M. Brau, Infrared Phys. 6 (1966) 183.
V. R. Panus and Z. U. Borisova, J. Appl. Chem. USSR 39 (1966) 937.
V. P. Shilo and B. T. Kolomiets, Bull. Acad. Sci. (USSR) Phys. Ser. 28 (1965) 1187.
B. T. Kolomiets and V. P. Shilo, “The Structure of Glass”, vol. 6, ed. E. A. Porai-Koshits. (English Translation published by Consultants Bureau, New York, 1966), pp. 187–188.
A. B. Chase and W. R. Wilcox, J. Amer. Ceram. Soc. 50 (1967) 332.
Rustum Roy, “Advances in Nucleation and Crystallization of Glasses”, eds. L. L. Hench and S. W. Freiman (Amer. Ceram. Soc. 1972), pp. 51–60.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Takamori, T., Roy, R. Effect of oxygen on structural properties of quenched Ge-Te melts. J Mater Sci 8, 415–422 (1973). https://doi.org/10.1007/BF00550163
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00550163