Abstract—
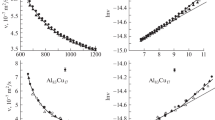
The devitrification, “cold” crystallization, and glass transition temperatures and melting point of samples in the B2O3–CaO–Al2O3–PbO system have been determined by differential thermal analysis. The viscosity of aluminum calcium borate melts containing up to 6.9% PbO has been measured in the temperature range 1153–1573 K. The results demonstrate that lead oxide additions reduce melt viscosity and that the density and surface tension of the melts increase with increasing lead oxide content and decrease with increasing temperature. High- and low-temperature regions have been identified where the melts have properties of Newtonian fluids. Cooling leads to polymerization and vitrification of the melts.





Similar content being viewed by others
REFERENCES
Nemilov, S.V., Viscosity of borate glass-forming melts: specific features of the BO4 tetrahedron as a kinetic unit, Glass Phys. Chem., 1997, vol. 23, no. 1, pp. 1–26.
Selivanov, E.N., Vusikhis, A.S., Sergeeva, S.V., Gulyaeva, R.I., and Ryabov, V.V., Thermal properties of glass and melts of the CaO – B2O3 – Al2O3 – CuO system, Glass Phys. Chem., 2021, vol. 47, no. 2, pp. 97–103.https://doi.org/10.1134/S1087659621020115
Klyuev, V.P. and Pevzner, B.Z., Thermal expansion and glass transition temperature of calcium borate and calcium aluminoborate glasses, Glass Phys. Chem., 2003, vol. 29, no. 2, pp. 127–136.https://doi.org/10.1023/A:1023498823701
Mohajerani, A., Martin, V., Boyd, D., and Zwanziger, J.W., On the mechanical properties of lead borate glass, J. Non-Cryst. Solids, 2013, vol. 381, pp. 29–34.https://doi.org/10.1016/j.jnoncrysol.2013.09.015
Bobkova, N.M., Borate glass as a key component of low-melting-point low-lead glazes, fluxes, and solders, Izv. Nats. Akad. Nauk Belarusi, Ser. Khim. Nauk, 2002, no. 4, pp. 14–17.
Eremyashev, V.E. and Osipova, L.M., Luminescence characteristics of alkali borate glasses, Steklo Keram., 2010, no. 10, pp. 22–24.
Pastukhov, E.A., Denisov, V.M., Bakhvalov, S.G., Physicochemical properties of fluxes intended for single-crystal growth of unstable compound semiconductors, in Fizicheskaya khimiya i tekhnologiya v metallurgii (Physical Chemistry and Technology in Metallurgy), Yekaterinburg, 1996, pp. 176–183.
Ye, N., Zhang, Y., Chen, W., Keszler, D., and Aka, G., Growth of nonlinear optical crystal Y0.57La0.72Sc2.71(BO3)4, J. Cryst. Growth, 2006, vol. 292, no. 2, pp. 464–467.https://doi.org/10.1016/j.jcrysgro.2006.04.055
Li, W., Huang, L., Zhang, G., and Ye, N., Growth and characterization of nonlinear optical crystal Lu0.66La0.95Sc2.39(BO3)4, J. Cryst. Growth, 2007, vol. 307, no. 2, pp. 405–409.https://doi.org/10.1016/j.jcrysgro.2007.07.017
Fedorova, M.V., Kononova, N.G., Kokh, A.E., and Shevchenko, V.S., Growth of MBO3 (M = La, Y, Sc) and LaSc3(BO3)4 crystals from LiBO2–LiF fluxes, Inorg. Mater., 2013, vol. 49, no. 5, pp. 482–486.https://doi.org/10.1134/S002016851304002X
Geodakyan, D.A., Petrosyan, B.V., Stepanyan, S.V., Vardanyan, R.A., and Geodakyan, K.D., Low-melting-point lead-containing glasses, Izv. NAN RA GIUA, Ser. TN, 2007, vol. 60, no. 3.
Babenko, A.A., Shartdinov, R.R., Upolovnikova, A.G., Smetannikov, A.N., and Gulyakov, V.S., Physical properties of CaO–SiO2–B2O3 slags containing 15% Al2O3 and 8% MgO, Steel Transl., 2019, vol. 49, no. 10, pp. 667–670.https://doi.org/10.3103/S0967091219100036
Kim, A.S., Akberdin, A.A., Sultangaziev, R.B., and Kireeva, G.M., Evaluation of the efficiency of application of high-basic boron-containing slags in melting of economically alloyed boron-containing steels, Metallurgist, 2018, nos. 1–2, pp. 34–38.https://doi.org/10.1007/s11015-018-0622-1
Belousov, A.A., Selivanov, E.N., Belyaev, V.V., and Litovskikh, S.N., Application of boron-containing fluxes for improving the quality of crude copper, Tsvetn. Metall., 2003, no. 10, pp. 13–17.
Denisov, V.M., Belousova, N.V., Istomin, S.A., Bakhvalov, S.G., and Pastukhov, E.A., Stroenie i svoistva rasplavlennykh oksidov (Structure and Properties of Molten Oxides), Yekaterinburg: Ural. Otd. Ross. Akad. Nauk, 1999.
Slag Atlas, Dusseldorf: Stahleisen, 1995, 2nd ed.
Rusakov, M.R., High-rate electrical melting and high-rate slag depletion processes, in Novye protsessy v metallurgii nikelya, medi i kobal’ta. Nauchnye trudy instituta Gipronikel’ (Novel Processes in Nickel, Copper, and Cobalt Metallurgy: Scientific Papers of Gipronikel Institute), Moscow: Ruda i Metally, 2000, pp. 126–138.
Biswas, K., Sontakke, A.D., Majumder, M., and Annapurna, K., Nonisothermal crystallization kinetics and microstructure evolution of calcium lanthanum metaborate glass, J. Therm. Anal. Calorim., 2010, vol. 101, pp. 143–151.https://doi.org/10.1007/s10973-009-0450-4
Morokhov, P.V., Anan’in, V.M., Ivannikov, A.A., Sevryukov, O.N., and Suchkov, A.N., Three-dimensional liquid–liquid immiscibility effect and its manifestations in viscometry and differential thermal analysis, Tsvetn. Met., 2014, no. 12, pp. 38–44.
Bubnova, R.S. and Filatov, S.K., Vysokotemperaturnaya kristallokhimiya boratov i borosilikatov (High-Temperature Crystal Chemistry of Borates and Borosilicates), St. Petersburg: Nauka, 2008.
Osipov, A.A., Osipova, L.M., and Bykov, V.M., Spektroskopiya i struktura shchelochnoboratnykh stekol i rasplavov (Spectroscopy and Structure of Alkali Borate Glasses and Melts), Yekaterinburg: Ural. Otd. Ross. Akad. Nauk, 2009.
Istomin, S.A., Khokhryakov, A.A., Ivanov, A.V., Chentsov, V.P., and Ryabov, V.V., Effect of lanthanide group REM oxides activated mechanically on the surface tension of borate melts, Russ. Metall. (Engl. Transl.), 2015, vol. 2015, no. 2, pp. 85–90.
Ivanov, A.V., Istomin, S.A., Khokhryakov, A.A., Chentsov, V.P., and Ryabov, V.V., Effect of the mechanical activation of Ln2O3 oxides (with Ln = Gd, Dy, Ho, and Lu) on the surface tension and density of borate melts, Russ. Metall. (Engl. Transl.), 2012, vol. 2012, no. 8, pp. 730–735.
Knyazyan, N.B., Detailed structure of borate and aluminoborate glasses, Khim. Zh. Arm., 2001, vol. 54, nos. 1–2, pp. 36–46.
Golubkov, V.V., Structural inhomogeneity of vitreous B2O3, Glass Phys. Chem., 1996, vol. 22, no. 3, pp. 178–185.
Chentsov, V.P., Shevchenko, V.G., Mozgowoi, A.G., and Pokrasin, M.A., Density and surface tension of heavy liquid-metal coolants: gallium and indium, Inorg. Mater.: Appl. Res., 2011, vol. 2, no. 5, pp. 468–473.https://doi.org/10.1134/S2075113311050108
Gladkikh, V.N., Viskozimetriya metallurgicheskikh rasplavov (Viscometry of Metallurgical Melts), Moscow: Metallurgiya, 1989.
Istomin, S.A., Ivanov, A.V., Ryabov, V.V., and Pastukhov, E.A., Effect of mechanical activation of rare-earth metal oxides on the viscosity of borate melts, Russ. Metall. (Engl. Transl.), 2011, no. 4, pp. 109–113.
Selivanov, E., Gulyaeva, R., Istomin, S., Belyaev, V., Tyushnyakov, S., and Bykov, A., Viscosity and thermal properties of slag in the process of autogenous smelting of copper–zinc concentrates, Trans. Inst. Min. Metall., Sect. C, 2015, vol. 124, no. 2, pp. 88–95.https://doi.org/10.1179/1743285514Y.0000000078
NETZSCH Proteus Software, Thermal Analysis, Version 4.8.3.
Metveenko, V.N. and Kirsanov, E.A., Structural viscosity and structural elasticity of polymer melts, Russ. J. Appl. Chem., 2018, vol. 91, no. 5, pp. 839–865.https://doi.org/10.1134/S1070427218050166
Guloyan, Yu.A., Fiziko-khimicheskie osnovy tekhnologii stekla (Physicochemical Principles of Glass Technology), Vladimir: Tranzit-IKS, 2008.
Mazelev, L.Ya., Boratnye stekla (Borate Glasses), Minsk: Akad. Nauk BSSR, 1958.
Funding
This work was supported by the Russian Foundation for Basic Research, project no. 18-29-24093 mk.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by O. Tsarev
Rights and permissions
About this article
Cite this article
Vusikhis, A.S., Sergeeva, S.V., Gulyaeva, R.I. et al. Structure-Sensitive Properties of Melts and Thermal Properties of Glasses in the B2O3–CaO–Al2O3–PbO System. Inorg Mater 58, 97–103 (2022). https://doi.org/10.1134/S0020168522010149
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0020168522010149