Summary
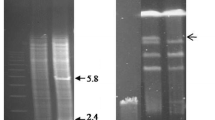
The Trichoderma harzianum imidazoleglycerolphosphate dehydratase gene (igh) has been isolated by complementation of a Saccharomyces cerevisiae his3 mutant using a direct expression vector. This Escherichia coli-yeast shuttle vector was developed to allow efficient cloning and expression of cDNA libraries. The cDNA is 627 nucleotides long and codes for a protein of 209 amino acids with an apparent molecular mass of 22 466 daltons. The predicted protein sequence showed 63.6%, 58.7%, and 38.4% identity respectively to the corresponding enzymes from S. cerevisiae, Pichia pastoris and E. coli. Northern analysis showed that the expression of the igh gene in T. harzianum is not inhibited by external histidine and the level of igh mRNA was about threefold higher in cells starved of histidine.
Similar content being viewed by others
References
Baker R (1987) Mycoparasitism: ecology and physiology. Can J Plant Pathol 9:370–376
Berlyn MB (1967) Gene-enzyme relationships in histidine biosynthesis in Aspergillus nidulans. Genetics 57:561–570
Brady DR, Houston LL (1973) Some properties of the catalytic sites of imidazoleglycerol phosphate dehydratase-histidinol phosphate phosphatase, a bifunctional enzyme from Salmonella typhimurium. J. Biol Chem 248:2588–2592
Burke ME, Pattee PA (1972) Histidine biosynthetic pathway in Staphylococcus aureus. Can J Microbiol 18:569–576
Carlomagno MS, Chiariotti L, Alifano P, Nappo AG, Bruni CB (1988) Structure and function of the Salmonella typhimurium and Escherichia coli K-12 histidine operons. J Mol Biol 203:585–606
Carsiotis M, Jones RF (1974) Cross pathway regulation: tryptophan-mediated control of histidine and arginine biosynthetic enzymes in Neurospora crassa. J. Bacteriol 119:889–892
Cesarini G, Murray JAH (1987) Plasmid vectors carrying the replication origin of filamentous single-stranded phages. In Setlow JK (ed) Genetic engineering, principles and methods, vol 9. Plenum, New York, pp 135–154
Chapman LF, Nester EW (1969) Gene-enzyme relationships in histidine biosynthesis in Bacillus subtilis. J Bacteriol 97:1444–1448
Chet I (1987) Trichoderma — application, mode of action, and potential as a biocontrol agent of soilborne plant pathogenic fungi. In: Chet I (ed) Innovative approaches to plant disease control. Wiley, New York, pp 137–160
Chet I (1990) Mycoparasitism — recognition, physiology and ecology. In: Baker RR, Dunn PE (eds) New Directions in biological control. Alternatives for suppressing agricultural pests and diseases. Liss, New York, pp 725–733
Chumley FG, Roth JR (1981) Genetic fusions that place the lactose genes under histidine operon control. J Mol Biol 145:697–712
Degrave W, Derynck R, Tavernier J, Haegeman G, Fiers W (1981) Nucleotide sequence of the chromosomal gene for human fibroblast (β1) interferon and of the flanking regions. Gene 14:137–143
Del Rey F, Garcia-Acha I, Nombela C (1979) The regulation of β-glucanase synthesis in fungi and yeast. J Gen Microbiol 110:83–89
Fani R, Bazzicalupo M, Damiani G, Bianchi A, Schipani C, Sgaramella V, Polsinelli M (1989) Cloning of histidine genes of Azospirillum brasilense: organization of the ABFH gene cluster and nucleotide sequence of the hisB gene. Mol Gen Genet 216:224–229
Fink GR (1964) Gene-enzyme relations in histidine biosynthesis in yeast. Science 146:525–527
Gatignol A, Baron M, Tiraby G (1987) Phleomycin resistance encoded by the ble gene from transposon Tn5 as a dominant selectable marker in Saccharomyces cerevisiae. Mol Gen Genet 207:342–348
Geremia R, Jacobs D, Goldman GH, Van Montagu M, Herrera-Estrella A (1991) Induction and secretion of hydrolytic enzymes by the biocontrol agent Trichoderma harzianum. In: Beemster ABR, Bollen GJ, Gerlagh M, Ruissen MA, Schippers B, Tempel A (eds) Biotic interactions and soil-borne diseases (Developments in Agricultural and Managed-Forest Ecology Series, vol 23). Elsevier, Amsterdam, pp 181–186
Glaser RD, Houston LL (1974) Subunit structure and photooxidation of yeast imidazoleglycerolphosphate dehydratase. Biochemistry 13:5145–5152
Goldman GH, Van Montagu M, Herrera-Estrella A (1990) Transformation of Trichoderma harzianum by high-voltage electric pulse. Curr Genet 17:169–174
Herrera-Estrella A, Goldman GH, Van Montagu M (1990) High-efficiency transformation system for the biocontrol agents, Trichoderma spp. Mol Microbiol 4:839–843
Hopwood DA, Bibb MJ, Chater KF, Kieser T, Bruton CJ, Kieser HM, Lydiate DJ, Smith CP, Ward JM, Schrempf H (1985) Genetic manipulation of Streptomyces. A laboratory manual. The John Innes Foundation, Norwich
Ito H, Fukuda Y, Murata K, Kimura A (1983) Transformation of intact yeast cells treated with alkali cations. J Bacteriol 153:163–168
Jones JDG, Dunsmuir P, Bedbrook J (1985) High level expression of introduced chimaeric genes in regenerated transformed plants. EMBO J 4:2411–2418
Kloos WE, Pattee PA (1965a) A biochemical characterization of histidine-dependent mutants of Staphylococcus aureus. J Gen Microbiol 39:185–194
Kloos WE, Pattee PA (1965b) Transduction analysis of the histidine region in Staphylococcus aureus. J Gen Microbiol 39:195–207
Legerton TL, Yanofsky C (1985) Cloning and characterization of the multifunctional his-3 gene of Neurospora crassa. Gene 39:129–140
Loper JC (1961) Enzyme complementation in mixed extracts of mutants from the Salmonella histidine B locus. Proc Natl Acad Sci USA 47:1440–1450
Maniatis T, Fritsch EF, Sambrook J (1982) Molecular cloning, a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
McKnight GL, Kato H, Upshall A, Parker MD, Saari G, O'Hara PJ (1985) Identification and molecular analysis of a third Aspergillus nidulans alcohol dehydrogenase gene. EMBO J 4:2093–2099
Okayama H, Berg P (1982) High-efficiency cloning of full-length cDNA. Mol Cell Biol 2:161–170
Prasad R, Niederberger P, Hutter R (1987) Tryptophan accumulation in Saccharomyces cerevisiae under the influence of an artificial yeast TRP gene cluster. Yeast 3:95–105
Revuelta JL, Jayaram M (1987) Phycomyces blakesleeanus TRP1 gene: organization and functional complementation in Escherichia coli and Saccharomyces cerevisiae. Mol Cell Biol 7:2664–2670
Schiestl RH, Gietz RD (1989) High efficiency transformation of intact yeast cells using single stranded nucleic acids as a carrier. Curr Genet 16:339–346
Schrank A, Tempelaars C, Sims PFG, Oliver SG, Broda P (1991) The trpC gene of Phanerochaete chrysosporium is unique in containing an intron but nevertheless maintains the order of functional domains seen in other fungi. Mol Microbiol 5:467–476
Sherman F, Fink GR, Hicks JB (1986) Laboratory course manual for methods in yeast genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY
Struhl K (1985) Nucleotide sequence and transcriptional mapping of the yeast pet56-his3-ded1 gene region. Nucleic Acids Res 13:8587–8601
Struhl K, Davis RW (1981) Transcription of the his3 gene region in Saccharomyces cerevisiae. J Mol Biol 152:535–552
Turgeon BG, MacRae WD, Garber RC, Fink GR, Yoder OC (1986) A cloned tryptophan-synthesis gene from the Ascomycete Cochliobolus heterostrophus functions in Escherichia coli, yeast and Aspergillus nidulans. Gene 42:79–88
Winkler ME (1987) Biosynthesis of histidine. In: Neidhardt FC, Ingraham JL, Low KB, Magasanik B, Schaechter M, Umbarger HE (eds) Escherichia coli and Salmonella typhimurium. Cellular and molecular biology, vol I American Society for Microbiology, Washington DC, pp 395–411
Wolfner M, Yep D, Messenguy F, Fink GR (1975) Integration of amino acid biosynthesis into the cell cycle of Saccharomyces cerevisiae. J Mol Biol 96:273–290
Yelton MM, Hamer JE, De Souza ER, Mullaney EJ, Timberlake WE (1983) Developmental regulation of the Aspergillus nidulans trpC gene. Proc Natl Acad Sci USA 80:7576–7580
Zhu J, Kempenaers W, Van Der Straeten D, Contreras R, Fiers W (1985) A method for fast and pure DNA elution from agarose gels by centrifugal filtration. Bio/Technology 3:1014–1016
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Goldman, G.H., Demolder, J., Dewaele, S. et al. Molecular cloning of the imidazoleglycerolphosphate dehydratase gene of Trichoderma harzianum by genetic complementation in Saccharomyces cerevisiae using a direct expression vector. Molec. Gen. Genet. 234, 481–488 (1992). https://doi.org/10.1007/BF00538709
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00538709