Abstract
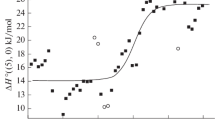
Starting from our previously proposed model of fused alkali metal halides, we discuss the processes involved in the mixing of fused alkali metal halides and dissolution (mixing) of TiCl4(g)[ZrCl4(liq)] in fused alkali metal halides and their mixtures. The resulting quantities are compared with the experimental. They vary with changes in the radius of the salt-solvent cation in the same direction as the experimental values. The dissolution of KClz in mixtures of fused alkali metal halides was considered from the point of view of the energy nonuniformity of the Me1 + and Me2 + cations.
It was shown that the experimentally observed increase in the enthalpy of mixing with increasing radius of the cation of the salt-solvent is caused not so much by a change in the bond energies of the KCl z−nn complexes in various media, as by a change in the bond energies of complex MeCl 3−4 anions of the solvent.
Similar content being viewed by others
References
M. V. Smirnov, O. M. Shabanov, and A. P. Khaimenov, Elektrokhimiya, 1, 1240, 1965.
M. I. Temkin, ZhFKh, 20, 105, 1946.
A. N. Kirgintsev and E. G. Avvakumov, Usp. khim, 34, 154, 1965.
M. V. Smirnov and A. P. Khaimenov, DAN SSSR, 158, 1172, 1965.
M. V. Smirnov and A. P. Khaimenov, Tr. In-ta Elektrokhimii UF SSSR, no. 7, 3, 1965.
N. F. Mott and M. J. Littleton, Trans. Farad. Soc., 34, 485, 1938.
A. Lidyard, Ionic Conductivity of Crystals, IL, Moscow, 1962.
M. Blander, F. F. Blankenship, and R. F. Newton, J. Phys. Chem., 63. 1259, 1959.
V. M. Smirnov and N. Ya. Chukreev, collection: Physical Chemistry of Fused Salts and Slags, Moscow, p. 227, 1962.
M. V. Smirnov, L. E. Ivanovskii, and N. A. Loginov, DAN SSSR, 121, 685, 1958.
M. V. Smirnov, V. E. Komarov, and A. N. Baraboshkin, Tr. In-ta Elektrokhimii UFAN SSSR, no. 2, 9, 1961.
O. V. Skiba and M. V. Smirnov, Tr. In-ta Elektrokhimii, UFAN SSSR, no. 2, 3, 1961.
M. V. Smirnov, Yu. N. Krasnov, and F. F. Khazemov, Tr. In-ta Elektrokhimii, UFAN SSSR, no. 5, 53, 1964.
M. V. Smirnov, Yu. S. Sokolovskii, and Yu. N. Krasnov, Tr. In-ta Elektrokhimii, UFAN SSSR, no. 5, 7, 1964.
M. V. Smirnov and O. A. Ryzhik, Tr. In-ta Elektrokhimii, UFAN SSSR, no. 6, 11, 1965.
M. V. Smirnov, V. S. Maksimov, and A. P. Khaimenov, ZhNKh, 9, 1765, 1966.
M. V. Smirnov and V. Ya. Kudyakov, ZhNKh, 10, 1211, 1965.
L. S. Hersh and O. J. Kleppa, J. Chem. Phys., 42, 1309, 1965.
L. S. Hersh and O. J. Kleppa, Trans. Farad. Soc., 59, 1850, 1963.
O. J. Kleppa, L. S. Hersh, and J. M. Toguri, Acta Chem. Scand., 17, 2575, 1963.
R. A. Gilbert, J. Phys. Chem., 67, 1143, 1963.
A. A. Vorob'ev, Physical Properties of Ionic, Crystalline Dielectrics, Izd-vo Tomskogo Universiteta, Tomsk, vol. 2, 1961.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Smirnov, M.V., Khaimenov, A.P. Enthalpies of mixing of fused salts. Theor Exp Chem 4, 40–44 (1970). https://doi.org/10.1007/BF00525945
Issue Date:
DOI: https://doi.org/10.1007/BF00525945