Abstract
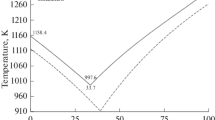
The fusibilities and liquidus temperatures of 10 compositions of the K2ZrF6–K2NbF7–KF–KCl quaternary system are determined in the component concentration ranges (mol %) 1.73–4.03 K2ZrF6, 2.33–4.55 K2NbF7, 48.62–65.37 KF, and 30.57–42.83 KCl. The studies are performed by differential thermal analysis on cooling. The dependence of the liquidus temperature on the molar ratios of the fluoride components of the molten phase is revealed. The compositions characterized by low molar ratios of potassium fluorozirconate to potassium fluoroniobate and simultaneously high molar ratios of potassium fluoride to the sum of zirconium and niobium salts have the lowest melting points. These ratios are 0.52–0.56 and 10.5–13.7, respectively. The liquidus temperatures of these compositions are in the range 854–873 K. High melting temperatures are characteristic of the melts that have the highest molar ratios of potassium fluorozirconate to potassium fluoroniobate and low molar ratios of potassium fluoride to the sum of the moles of zirconium and niobium salts. These ratios are 5.4–7.9 and 0.8–1.1, respectively. The liquidus temperatures of these compositions are 920–974 K.
Similar content being viewed by others
REFERENCES
L. G. Nekhamkin, Metallurgy of Zirconium and Hafnium (Metallurgiya, Moscow, 1979).
L. P. Polyakova and P. T. Stangrit, “Homogeneity of zirconium–niobium alloys of various genesis,” in Chemistry, Chemical Technology, and Metallurgy of Rare Elements (Apatity, 1982), p. 135.
V. I. Konstantinov, Electrolytic Production of Tantalum, Niobium, and Their Alloys (Metallurgiya, Moscow, 1977).
G. W. Mellors and S. Senderoff, “Electrodeposition of coherent deposits of refractory metals. I. Niobium,” J. Electrochem. Soc. 112, 266–272 (1965).
L. E. Ivanovskii and M. T. Krasil’nikov, “Electrode processes and the effect of oxygen during the electrolytic deposition of niobium from potassium fluoroniobate,” Trudy Inst. Elektrokhim. UFAN SSSR, No. 1, 49–54 (1960).
L. E. Ivanovskii and O. S. Petenev, “Some processes during the cathodic deposition of zirconium from chloride–fluoride melts,” Trudy Inst. Elektrokhim. UFAN SSSR, No. 2, 71–78 (1961).
K. P. Lebedeva and A. N. Baraboshkin, “Influence of electrolysis conditions on the structure of zirconium deposit. I. Electrolysis of chloride melts containing tetravalent zirconium,” Trudy Inst. Elektrokhim. UFAN SSSR, No. 6, 93–99 (1965).
K. P. Lebedeva and A. N. Baraboshkin, “Influence of electrolysis conditions on the structure of zirconium precipitation. II. Influence of fluorine ions on the structure of zirconium deposit,” Trudy Inst. Elektrokhim. UFAN SSSR, No. 6, 101–106 (1965).
A. N. Baraboshkin and K. P. Lebedeva, “Influence of electrolysis conditions on the structure of zirconium deposit,” Trudy Inst. Elektrokhim. UFAN SSSR, No. 7, 59–67 (1965).
V. A. Kotelevskii, F. V. Kovalev, L. E. Ivanovskii, Yu. U. Samson, F. N. Kozlov, and I. A. Baranov, “Production of niobium coatings by electrolysis of molten media,” Trudy Inst. Elektrokhim. UFAN SSSR, No. 21, 56–60 (1974).
K. I. Trifonov and V. I. Medvedev, “Fusibility of the salt mixtures containing zirconium and hafnium tetrachlorides and potassium tetrachloraluminate,” Rasplavy, No. 6, 87–89 (2006).
A. B. Salyulev, E. G. Vovkotrub, and V. N. Strekalovskii, “Interaction of zirconium and hafnium tetrachlorides with cesium, rubidium, and potassium chlorides and the Raman spectra of reaction products,” Rasplavy, No. 3, 45–49 (2008).
K. I. Trifonov, A. S. Larionov, V. E. Krotov, and A. F. Nikiforov, “Viscosity of the salt melts of the KAlCl4–ZrCl4–HfCl4 system,” Rasplavy, No. 2, 113–117 (2012).
A. Barhoun, Y. Berghoute, and F. Lantelme, “Electrodeposition of niobium from fluoroniobate K2NbF7 in fused NaCl–KCl,” J. Alloys Compd., 241–252 (1992).
C. Decroly and R. Winand, “Électrodéposition en bain de sels fondus de poudres d’alliages zirconium–niobium,” J. Less Comm. Metals. 6, 132–151 (1964).
A. Sheikh, R. Winand, and A. Fontana, “Production of zirconium metal by electrolysis in molten fluorides baths, the cell being fed by tablets of zirconium oxide and carbon,” J. Nucl. Mater. 39, 84–92 (1971).
V. I. Konstantinov, E. G. Polyakov, and P. T. Stangrit, “Cathodic processes at electrolysis of chloride–fluoride and oxyfluoride melts of niobium,” Electrochim. Acta 26, 445–448 (1981).
L. P. Polyakova and P. T. Stangrit, “Cathodic processes at electrolysis of chlorine and chloride–fluoride melts of zirconium,” Electrochim. Acta. 11, 1641–1645 (1992).
Z. Alimova, E. Polyacov, and V. Kremenetskiy, “The role of fluoride ions in reduction–oxidation equilibrium in CsCl–KCl–NaCl–K2NbF7 melts,” J. Fluorine Chem. 2, 203–209 (1992).
V. Van, A. Silny, and V. Danek, “Electrochemical study of niobium fluoride and oxyfluoride complexes in molten LiF–KF–K2NbF7 bath,” Electrochem. Commun. 1, 295–300 (1999).
D. Quaranta, L. Massot, M. Gibilaro, E. Mendes, J. Serp, and P. Chamelot, “Zirconium(IV) electrochemical behavior in molten LiF–NaF,” Electrochim. Acta. 265, 586–593 (2018).
Ch. Li, Sh. Li, Y. Che, et al., “Electrochemical behavior of niobium ions in molten KCl–NaCl,” J. Mater. Res. Technol. 9, 9341–9347 (2020).
S. F. Katyshev, V. N. Desyatnik, and L. M. Teslyuk, “Physicochemical properties of alkali metal and zirconium fluoride melts,” Tsvetn. Met., No. 8, 103–105 (2006).
S. F. Katyshev, L. M. Teslyuk, N. N. Kurbatov, L. V. Semeikina, and A. V. Shchepin, “Properties of possible electrolytes for the production of metallic zirconium,” Vestn. UGTU-UPI. Teoriya Praktika Elektrokhim. Protsessov, No. 14, 98–103 (2004).
M. Chrenkova, V. Danielik, B. Kubikova, V. Daněk, “CALPHAD: phase diagram of the system LiF–NaF–K2NbF7,” Calphad 27 (1), 19–23 (2003).
J. Mlynarikova, M. Boča, and L. Kipsova, “The role of the alkaline cations in the density and volume properties of the melts MF–K2NbF7 (MF = LiF–NaF, LiF–KF and NaF–KF),” J. Mol. Liq. 140 (1–3), 101–107 (2008).
K. I. Trifonov and S. V. Afanas’ev, “Properties of the chlorination products of the waste of ferroniobium production,” in Proceedings of Congress on Fundamental Studies and Applied Development of Processing and Recycling of Technical Products (Yekaterinburg, 2017), pp. 348–349.
K. I. Trifonov and G. N. Titov, “Influence of sodium chloride on the solidification temperature and electrical conductivity of the electrolyte for the electrolysis of zirconium,” in Proceedings of VII Kola Seminar on Electrochemistry of Rare and Nonferrous Metals (Apatity, 1992), p. 116.
G. N. Titov, A. A. Red’kin, and P. I. Moskalenko, “Influence of the chloride–fluoride melt electrolysis conditions on the phase composition during the production of zirconium in industrial skull electrolyzers,” in Proceedings of VIII Kola Seminar on Electrochemistry of Rare Metals (Apatity, 1995), pp. 71–72.
K. I. Trifonov, S. F. Katyshev, and A. F. Nikiforov, “Spent electrolyte of zirconium production as a raw material for producing zirconium–niobium alloys,” in Proceedings of Congress on Fundamental Investigations and Applied Development of the Processing and recycling of Technical Products (Yekaterinburg, 2017), pp. 347–348.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors declare that they have no conflicts of interest.
Additional information
Translated by K. Shakhlevich
Rights and permissions
About this article
Cite this article
Trifonov, K.I., Krotov, V.E., Nikiforov, A.F. et al. Fusibility of the K2ZrF6–K2NbF7–KF–KCl System. Russ. Metall. 2022, 955–957 (2022). https://doi.org/10.1134/S0036029522080225
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0036029522080225