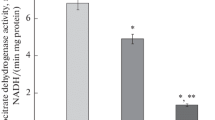
Summary
Activities (μmol x min−1 x g liver) and zonal distributions of key enzymes of carbohydrate metabolism were studied in livers of streptozotocin-diabetic rats and compared to the values in alloxan-diabetes.
-
1.
Streptozotocin led to a non-ketotic diabetes with blood glucose being increased by more than fivefold but ketone bodies being in the normal range, while alloxan produced a ketotic diabetes with blood glucose, acetoacetate and β-hydroxybutyrate being elevated by more than fivefold.
-
2.
Portal insulin was decreased to about 20% in streptozotocin- and more drastically to about 7% in alloxan-diabetes. Conversely, portal glucagon was increased in the two states to about 250% and 180%, respectively.
-
3.
The glucogenic key enzyme phosphoenolpyruvate carboxykinase (PEPCK) was enhanced in streptozotocinand alloxan-diabetes to over 300%, while the glycolytic pyruvate kinase L (PKL) was lowered to 65% and 80%, respectively. The normal periportal to perivenous gradient of PEPCK of about 3:1, as measured in microdissected tissue samples, was maintained with elevated activities in the two zones. The normal periportal to perivenous gradient of PKL of 1:1.7 was diminished with lowered activities in the two zones.
-
4.
The glucogenic glucose-6-phosphatase (G6Pase) was increased in streptozotocin- and alloxan-diabetes to 130% and 140%, respectively, while the glucose utilizing glucokinase (GK) was decreased to 60% and 50%, respectively. The normal periportal to perivenous gradient of G6Pase, demonstrated histochemically, remained unaffected.
-
5.
Carnitine palmitoyltransferase (CPT) was increased to over 190% and acetyl-CoA carboxylase (ACC) was decreased to 60% in streptozotocin, non-ketotic diabetes, while the two enzymes were altered more drastically to 400% and 50%, respectively, in alloxan, ketotic diabetes.
The results indicate that both in the ketotic and nonketotic diabetic state the gluconeogenic capacity of the liver was increased mainly in the periportal zone and the glycolytic capacity was decreased mainly in the perivenous area. In both types of diabetes the glucostat function of the liver with its typical reciprocal zonal distribution of glucogenic and glycolytic enzymes was not lost, but only impaired owing to shifts of the enzyme levels in the periportal and perivenous zone. This quantitative rather than qualitative alteration would be in accord with the requirement for the liver persisting in both non-ketotic and ketotic diabetes to handle excess nutritional glucose at least in part.
Similar content being viewed by others
References
Andersen B, Nath A, Jungermann K (1982) Heterogeneous distribution of phosphoenolpyruvate carboxykinase in rat liver parenchyma, isolated and cultured hepatocytes. Eur J Cell Biol 28:47–53
Andersen B, Zierz S, Jungermann K (1984) Alteration in zonation of succinate dehydrogenase, phosphoenolpyruvate carboxykinase and glucose-6-phosphatase in regenerating rat liver. Histochemistry 80:97–101
Balks HJ, Jungermann K (1984) Regulation of peripheral insulin/glucagon levels by rat liver. Eur J Biochem 141:645–650
Baxter LCA, Schofield PJ (1980) The effects of a high fat diet on chronic streptozotocin-diabetic rats. Diabetologia 18:239–245
Brinkmann A, Katz N, Sasse D, Jungermann K (1978) Increase of the gluconeogenic and decrease of the glycolytic capacity of rat liver with a change of the metabolic zonation after partial hepatectomy. Hoppe Seyler's Z Physiol Chem 359:1561–1571
Chatzipanagiotou S, Nath A, Vogt B, Jungermann K (1985) Alteration in the capacities as well as in the zonal and cellular distributions of pyruvate kinase L and M2 in regenerating rat liver. Biol Chem Hoppe-Seyler 366:271–280
Crisp DM, Pogson CI (1972) Glycolytic and gluconeogenic enzyme activities in parenchymal and non-parenchymal cells from mouse liver. Biochem J 126:1009–1023
Garfield SA, Cardell RR (1979) Hepatic glucose-6-phosphatase activities and correlated ultrastructural alterations in hepatocytes of diabetic rats. Diabetes 28:664–679
Guder W, Schmidt U (1976) Liver cell heterogeneity: The distribution of pyruvate kinase and phosphoenolpyruvate carboxykinase in the liver lobule of fed and starved rats. Hoppe-Seyler's Z Physiol Chem 357:1793–1800
Hartmann H, Beckh K, Jungermann K (1982) Direct control of glycogen metabolism in the perfused rat liver by the sympathetic innervation. Eur J Biochem 123:521–526
Häussinger D (1983) Hepatocyte heterogeneity in glutamine and ammonia metabolism and the role of an intracellular glutamine cycle during ureagenesis in perfused rat liver. Eur J Biochem 133:269–275
Hers HG, Hue L (1983) Gluconeogenesis and related aspects of glycolysis. Annu Rev Biochem 52:617–653
Hue L, van de Werve G (1981) Short-term regulation of liver metabolism. Elsevier/North Holland, Amsterdam
Höppner W, Süssmuth W, Seitz HJ (1985) Effect of thyroid state on cyclic AMP-mediated induction of hepatic phosphoenolpyruvate carboxykinase. Biochem J 226:67–73
Jungermann K (1985) Metabolische Zonierung des Leberparenchyms. Bedeutung für die Regulation des Glucostaten Leber. Naturwissenschaften 72:76–84
Jungermann K (1986a) Metabolic zonation of liver parenchyma: Significance for the regulation of glycogen metabolism, gluconeogenesis and glycolysis. Diabetes/Metabolism Rev in press
Jungermann K (1986b) Dynamics of zonal hepatocyte heterogeneity. Perinatal development and adaptive alterations during regeneration after partial hepatectomy, starvation and diabetes. Acta Histochem (Suppl) 32:89–98
Jungermann K, Katz N (1982) Functional hepatocellular heterogeneity. Hepatology 2:385–395
Jungermann K, Sasse D (1978) Heterogeneity of liver parenchymal cells. Trends Biochem Sci 3:198–202
Jungermann K, Heilbronn R, Katz N, Sasse D (1982) The glucose/glucose-6-phosphate cycle in the periportal and perivenous zone of rat liver. Eur J Biochem 123:429–436
Katz N, Nauck M, Wilson P (1979) Induction of glucokinase by insulin under the permissive action of dexamethasone in primary rat hepatocyte cultures. Biochem Biophys Res Commun 88:23–29
Katz N, Teutsch H, Sasse D, Jungermann K (1977) Heterogeneous distribution of glucose-6-phosphatase in periportal and perivenous rat liver tissue. FEBS Lett 76:226–230
Katz N, Fischer W, Giffhorn S (1983) Distribution of enzymes of fatty acid and ketone body metabolism in periportal and perivenous rat liver tissue. Eur J Biochem 135:103–107
Lautt WW (1983) Afferent and efferent neural roles in liver function. Prog Neurobiol 21:323–348
Lemieux G, Arando MR, Fournel P, Lemieux C (1984) Renal enzymes during experimental diabetes mellitus in the rat. Role of insulin, carbohydrate metabolism and ketoacidosis. Can J Physiol Pharmacol 62:70–75
Lojda Z, Gossrau R, Schiebler TH (1976) Enzym-histochemische Methoden. Springer, Berlin Heidelberg New York, p 231
Loose D, Cameron K, Short H, Hanson R (1985) Thyroid hormone regulates transcription of the gene for cytosolic phosphoenolpyruvate carboxykinase (GTP) in rat liver. Biochemistry 24:4509–4512
Lowry OH, Passonneau JV (1972) A flexible system of enzymatic analysis. Academic Press, New York
Matsumura T, Thurman RG (1984) Predominance of glycolysis in pericentral regions of the liver lobule. Eur J Biochem 140:229–234
Matsumura T, Kashiwagi T, Meren H, Thurman RG (1984) Gluconcogenesis predominates in periportal regions of the liver lobule. Eur J Biochem 144:409–415
Miethke H, Wittig B, Nath A, Zierz S, Jungermann K (1985) Metabolic zonation in liver of diabetic rats. Zonal distribution of phosphoenolpyruvate carboxykinase, pyruvate kinase, glucose-6-phosphatase and succinate dehydrogenase. Biol Chem Hoppe-Seyler 366:493–501
Nauck M, Wölfle D, Katz N, Jungermann K (1981) Modulation of the glucagon-dependent induction of phosphoenolpyruvate carboxykinase and tyrosine aminotransferase by arterial and venous oxygen concentrations in hepatocyte cultures. Eur J Biochem 119:657–661
Nuber R, Teutsch HF, Sasse D (1980) Metabolic zonation in thioacetamide-induced liver cirrhosis. Histochemistry 69:277–288
Probst I, Jungermann K (1983) The glucagon-insulin antagonism and glucagon-dexamethasone synergism in the induction of phosphoenolpyruvate carboxykinase in cultured rat hepatocytes. Hoppe-Seyler's Z Physiol Chem 364:1739–1764
Probst I, Schwartz P, Jungermann K (1982) Induction in primary culture of “gluconeogenic” and “glycolytic” hepatocytes resembling periportal and perivenous cells. Eur J Biochem 126:271–278
Sasaki K, Cripe PT, Koch SR, Andreone TL, Petersen DD, Beate EG, Granner D (1984) Multihormonal regulation of phosphoenolpyruvate carboxykinase gene transcription. J Biol Chem 259:15242–15251
Schudt C (1979) Hormonal regulation of glucokinase in primary cultures of rat hepatocytes. Eur J Biochem 98:77–82
Seubert W, Huth W (1965) On the mechanism of gluconeogenesis and its regulation. II. The mechanism of gluconcogenesis from pyruvate and fumarate. Biochem Z 343:179–191
Shimazu T (1983) Reciprocal innervation of the liver: Its significance in metabolic control. Adv Metabol Disorders 10:355–384
Süssmuth W, Höppner W, Seitz HJ, Luda D, Harneit A (1984) Permissive action of thyroid hormones in the cAMP-mediated induction of phosphoenolpyruvate carboxykinase in hepatocytes in culture. Eur J Biochem 143:607–611
Wittig B, Zierz S, Gubernatis G, Nath A, Jungermann K (1985) Glucostat capacity and metabolic zonation in rat liver after portocaval anastomosis. Biol Chem Hoppe-Seyler 366:713–722
Wolf HPO, Engel DW (1985) Decrease of fatty acid oxidation, ketogenesis and gluconeogenesis in isolated perfused rat liver by phenylalkyl oxirane carboxylate (B 807-27) due to inhibition of CPT I (EC 2.3.1.21). Eur J Biochem 146:359–363
Wölfle D, Hartmann H, Jungermann K (1981) Induction of phosphoenolpyruvate carboxykinase by sympathetic agents in primary cultures of adult rat hepatocytes. Biochem Biophys Res Commun 98:1084–1090
Zierz S, Katz N, Jungermann K (1983) Distribution of pyruvate kinase type L and M2 in microdissected periportal and perivenous rat liver tissue with different dietary states. Hoppe-Seyler's Z Physiol Chem 364:1447–1453
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Miethke, H., Wittig, B., Nath, A. et al. Gluconeogenic-glycolytic capacities and metabolic zonation in liver of rats with streptozotocin, non-ketotic as compared to alloxan, ketotic diabetes. Histochemistry 85, 483–489 (1986). https://doi.org/10.1007/BF00508430
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00508430