Summary
The purpose of the present study was to further characterize the inhibition by prostaglandin E2 (PGE2) of adrenocorticotropin (ACTH) and β-endorphin release from rat anterior pituitary fragments in vitro. Peptide hormone release was induced by vasopressin, which initiates secretion via cell surface receptors, or by secretagogues which can mimic various post-receptor mechanisms and the effect of PGE2 was examined.
-
1.
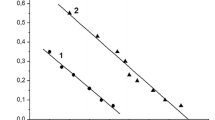
Concentration-response curves of the effect of vasopressin on the release of β-endorphin-like (β-End-IR) and ACTH-like immunoreactivity (ACTH-IR) were constructed in the absence or presence of a fixed concentration of PGE2. The concentration-response curve of vasopressin was shifted to the right about 8-fold by PGE2 (1 μmol/l) without altering the maximum effect.
-
2.
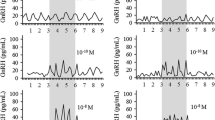
PGE2 (60 nmol/l–1 μmol/l) markedly reduced β-End-IR release induced by 8-bromoadenosine-3′,5′-cyclicmonophosphate (8Br-cAMP) (1 mmol/l). Omission of Ca2+ from the incubation medium did not prevent PGE2-induced inhibition of 8Br-cAMP-evoked secretion.
-
3.
4β-Phorbol, 12β-myristate, 13α-acetate (PMA) stimulated β-End-IR and ACTH-IR release in a concentration-dependent manner. This effect was not blocked by indometacin or eicosatetraynoic acid. PG E2 (>100 nmol/l) reduced PMA (100 nmol/l)-elicited secretion by about 50%.
-
4.
PG E2 (1 μmol/l) almost halved β-End-IR release caused by K+ (30 mmol/l).
-
5.
After pre-incubation in Ca2+-free medium, re-introduction of Ca2+ (1.3 mmol/l) elicited β-End-IR release. This response was abolished by PG E2 (1 μmol/l).
-
6.
The addition of Ba2+ (10 mmol/l) to a Ca2+-free medium markedly enhanced β-End-IR release. The stimulation by Ba2+ was blocked by elevating the Ca2+ concentration to 15.3 mmol/l. PGE2 (1 μmol/l) did not influence the Ba2+-induced secretion.
These data suggest that PGE2 inhibits receptor-mediated stimulation of β-End-IR/ACTH-IR release in an apparently competitive manner. We conclude that the ability of PGE2 to inhibit secretion from corticotrophs is not linked to a particular post-receptor mechanism but depends on the interference with a mechanism which follows second messenger formation and which, furthermore, is crucial for exocytotic release mechanisms, e.g. the intracellular availability of calcium.
Similar content being viewed by others
References
Axelrod J, Reisine TD (1984) Stress hormones: Their interaction and regulation. Science 224:452–459
Canonico PL, Valdenegro CA, Judd AM, MacLeod RM (1984) Arachidonic acid metabolism and thyrotropin secretion in vitro. Eur J Pharmacol 98:45–52
Canonico PL, Judd AM, Koike K, Valdenegro CA, MacLeod RM (1985) Arachidonate stimulates prolactin release in vitro: A role for the fatty acid and its metabolites as intracellular regulator(s) in mammotrophs. Endocrinology 116:218–225
Catt KJ, Loumaye E, Wynn P, Suarez-Quian C, Kiesel L, Iwashita M, Hirota K, Morgan R, Chang J (1984) Receptor-mediated activation mechanisms in the hypothalamic control of pituitary-gonadal function. In: Labrie F, Proulx L (eds) Endocrinology. Elsevier Science Publishers BV, Amsterdam, pp 57–65
Denzlinger C, Hertting G, Jackisch R (1982) Synaptosomal calcium uptake systems: prostaglandins are probably not involved in the regulation of calcium fluxes into and within the nerve endings. J Neurochem 39:499–506
Douglas WW, Rubin RP (1964) The effects of alkaline earths and other divalent cations on adrenal medullary secretion. J Physiol (Lond) 175:231–241
Douglas WW, Taraskevich PS, Tomiko SA (1983) Secretagogue effect of barium on output of melanocyte-stimulating hormone from pars intermedia of the mouse pituitary. J Physiol (Lond) 338:243–257
Duniec Z, Robak J, Gryglewski R (1983) Antioxidant properties of some chemicals vs their influence on cyclooxygenase and lipoxidase activities. Biochem Pharmacol 32:2283–2286
Gill DL, Grollman EF, Kohn LD (1981) Calcium transport mechanisms in membrane vesicles from guinea pig brain synaptosomes. J Biol Chem 256:184–192
Hedge GA (1976) Hypothalamic and pituitary effects of prostaglandins on ACTH secretion. Prostaglandins 11:293–301
Hedge GA (1977) Stimulation of ACTH secretion by indomethacin and reversal by exogenous prostaglandins. Prostaglandins 14:145–151
Hedqvist P (1977) Basic mechanisms of prostaglandin action on autonomic neurotransmission. Annu Rev Pharmacol Toxicol 17:259–279
Heisler S (1984) 12-O-Tetradecanoyl-phorbol-13-acetate-induced ACTH secretion in pituitary tumor cells. Eur J Pharmacol 98:177–183
Hess P, Tsien RW (1984) Mechanism of ion permeation through calcium channels. Nature 309:453–456
Holmes MC, Antoni FA, Szentendrei T (1984) Pituitary receptors for corticotropin-releasing factor: No effect of vasopressin on binding or activation of adenylate cyclase. Neuroendocrinology 39:162–169
Judd AM, Koike K, MacLeod RM (1985) GRF increases release of growth hormone and arachidonate from anterior pituitary cells. Am J Physiol 248:E438-E442
Kaczorowski GJ, Costello L, Dethmers J, Trumble MJ, Vandlen RL (1984) Mechanism of Ca2+ transport in plasma membrane vesicles prepared from cultured pituitary cells. J Biol Chem 259:9395–9403
Kim RS, LaBella FS (1985) Calcium translocation by fatty acid derivatives in a two-phase partition model. Structure-activity relationships. Biochim Biophys Acta 833:386–395
Knepel W, Meyen G (1986) Effect of various blockers of arachidonic acid metabolism on release of beta-endorphin- and adrenocorticotropin-like immunoreactivity induced by phospholipase A2 from rat adenohypophysis in vitro. Neuroendocrinology (in press)
Knepel W, Nutto D, Anhut H (1983) β-Endorphin controls vasopressin release during foot shock-induced stress in the rat. Regul Pept 7:9–19
Knepel W, Homolka L, Vlaskovska M, Nutto D (1984) In vitro adrenocorticotropin/β-endorphin-releasing activity of vasopressin analogs is related neither to pressor nor to antidiuretic activity. Endocrinology 114:1797–1804
Knepel W, Nutto D, Vlaskowska M, Kittel Ch (1985a) Inhibition by prostaglandin E2 of the release of vasopressin and β-endorphin from rat pituitary neurointermediate lobe or medial basal hypothalamus in vitro. J Endocrinol 106:189–195
Knepel W, Przewlocki R, Nutto D, Herz A (1985b) Foot shock stress-induced release of vasopressin in adenohypophys-ectomized and hypophysectomized rats. Endocrinology 117: 292–299
Knepel W, Schwaninger M, Döhler KD (1985c) Corelease of dynorphin-like immunoreactivity, luteinizing hormone, and follicle-stimulating hormone from rat adenohypophysis in vitro. Endocrinology 117:481–487
Knepel W, Vlaskovska M, Meyer DK (1985d) Release of prostaglandin E2 and β-endorphin-like immunoreactivity from rat adenohypophysis in vitro: Variations after adrenalectomy or lesions of the paraventricular nuclei. Brain Res 326:87–94
Kolesnick RN, Gershengorn MC (1985) Arachidonic acid inhibits thyrotropin-releasing hormone-induced elevation of cytoplasmic free calcium in GH3 pituitary cells. J Biol Chem 260:707–713
Kolesnick RN, Musacchio I, Thaw C, Gershengorn MC (1984) Arachidonic acid mobilizes calcium and stimulates prolactin secretion from GH3 cells. Am J Physiol 246:E458-E462
Lorenson MY, Robson DL, Jacobs LS (1983) Divalent cation inhibition of hormone release from isolated adenohypophysial secretory granules. J Biol Chem 258:8618–8622
Lynch CJ, Charest R, Bocckino SB, Exton JH, Blackmore PF (1985) Inhibition of hepatic α1-adrenergic effects and binding by phorbol myristate acetate. J Biol Chem 260:2844–2851
Majerus PW, Wilson DB, Connolly TM, Bross TE, Neufeld EJ (1985) Phosphoinositide turnover provides a link in stimulus-response coupling. Trends Biochem Sci 10:168–171
Marone G, Kagey-Sobotka A, Lichtenstein LM (1979) Effects of arachidonic acid and its metabolites on antigen-induced histamine release from human basophils in vitro. J Immunol 123:1669–1677
Marshall PJ, Dixon JF, Hokin LE (1980) Evidence for a role in stimulus-secretion coupling of prostaglandins derived from release of arachidonoyl residues as a result of phosphatidylinositol breakdown. Proc Natl Acad Sci USA 77:3292–3296
Metz SA, Fujimoto WY, Robertson RP (1982) Lipoxygenation of arachidonic acid: a pivotal step in stimulus-secretion coupling in the pancreatic beta cell. Endocrinology 111:2141–2143
Mo N, Ammari R, Dun NJ (1985) Prostaglandin E1 inhibits calcium-dependent potentials in mammalian sympathetic neurons. Brain Res 334:325–329
Nishizuka Y (1984) The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature 308:693–698
Raymond V, Leung PCK, Velleux R, Labrie F (1985) Vasopressin rapidly stimulates phosphatidic acid-phosphatidylinositol turnover in rat anterior pituitary cells. FEBS Lett 182:196–200
Smith JB, Smith L (1984) Rapid calcium mobilization by vasopressin and prostaglandin F2α is independent of sodium influx in quiescent 3T3 cells. Biochem Biophys Res Commun 123:803–809
Snyder GD, Capdevila J, Chacos N, Manna S, Falck JR (1983) Action of luteinizing hormone-releasing hormone: Involvement of novel arachidonic acid metabolites. Proc Natl Acad Sci USA 80:3504–3507
Stjärne L (1979) Role of prostaglandins and cyclic adenosine monophate in release. In: Paton DM (ed) The release of catecholamines from adrenergic neurons. Pergamon Press, Oxford, pp 111–142
Sullivan TJ, Parker CW (1979) Possible role of arachidonic acid and its metabolites in mediator release from rat mast cells. J Immunol 122:431–436
Surprenant A (1982) Correlation between electrical activity and ACTH/β-endorphin secretion in mouse pituitary tumor cells. J Cell Biol 95:559–566
Taraskevich PS, Douglas WW (1984) Electrical activity in adenohypophyseal cells and effects of hypophyseotropic substances. Fed Proc 43:2373–2378
Thaw CN, Raaka EG, Gershengorn MC (1984) Evidence that cobalt ion inhibition of prolactin secretion occurs at an intracellular locus. Am J Physiol 247:C150-C155
Yale W, Rivier C (1977) Substances modulating the secretion of ACTH by cultured anterior pituitary cells. Fed Proc 36:2094–2099
Vlaskovska M, Knepel W (1984) Beta-endorphin and adrenocorticotropin release from rat adenohypophysis in vitro: Evidence for local modulation by arachidonic acid metabolites of the cyclooxygenase and lipoxygenase pathway. Neuroendocrinology 39:334–342
Vlaskovska M, Hertting G, Knepel W (1984) Adrenocorticotropin and β-endorphin release from rat adenohypophysis in vitro: Inhibition by prostaglandin E2 formed locally in response to vasopressin and corticotropin-releasing factor. Endocrinology 115:895–903
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Knepel, W., Götz, D. Effect of prostaglandin E2 on ACTH and β-endorphin release from rat adenohypophysis in vitro after secretagogues which can mimic various first or second messengers. Naunyn-Schmiedeberg's Arch. Pharmacol. 333, 149–155 (1986). https://doi.org/10.1007/BF00506518
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00506518