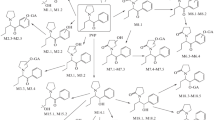
Summary
A series of six major urinary conjugates in the metabolism of antipyrine in man and rat has been investigated. A preparative isolation procedure has been developed using chromatography of methanolic extracts from urine on silica gel. In a two-step chromatographic procedure, methanolic extracts are first separated in 1) a “free fraction”, containing unconjugated phase-I-metabolites and unchanged antipyrine, 2) a sulfate fraction and 3) a glucuronide fraction. Sulfate and glucuronide fraction, respectively, are each subjected to a second run for separation into their three components. Thus, the following conjugates have been prepared: 4-hydroxy-antipyrine sulfate, norantipyrine sulfate, 4,4′-dihydroxy-antipyrine sulfate, and 4-hydroxy-antipyrine glucuronide, porantipyrine glucuronide, 3-hydroxymethyl-antipyrine glucuronide. Methodology is also applicable to bile fluid and liver perfusate.
Stability of isolated conjugates against acid hydrolysis has been studied to show that strongly marked differences exist in this series of conjugates. Field desorption mass spectrometry has been used for the direct identification of intact conjugates in an underivatized form.
Using 13C-NMR, the structure of norantipyrine glucuronide has been established as an 5-enol glucuronide. By analogy, a structure of 5-enol sulfate is proposed for norantipyrine sulfate.
From a semiquantitative examination by TLC of urine extracts from man and rat, it becomes apparent, that in the rat, at the dose level studied, sulfate formation is the predominant conjugation pathway. In man, glucuronides are the most prominent type of conjugates. Formation of sulfates is minimal up to a dose of 15 mg/kg antipyrine.
Similar content being viewed by others
References
Aitio A (1978) Conjugation reactions in drug biotransformation Elsevier/North-Holland Biomedical Press, Amsterdam
Bässmann H, Böttcher J (1980) New phase-I and phase-II-metabolites of phenazone. Naunyn-Schmiedeberg's Arch Pharmacol 311 Suppl: R-17
Bässmann H, Böttcher J, Schüppel R (1979) 4,4′-Dihydroxyphenazone as an urinary metabolite of phenazone in different species including man. Naunyn-Schmiedeberg's Arch Pharmacol 309:203–205
Böttcher J, Bässmann H, Schüppel R (1979) Quantitation of metabolism of phenazone in the rat and in the isolated perfused rat liver preparation. Naunyn-Schmiedeberg's Arch Pharmacol 307 Suppl: R-8
Böttcher J, Bässmann H, Schüppel R (1981a) Modification of hydroxylation and conjugation pattern of antipyrine by various inducers of MFO in the rat. Proc 5th Int Sympos on Microsomes and Drug Oxidations. Tokyo
Böttcher J, Bässmann H, Schüppel R (1981b) Direct quantitation of urinary conjugates in 3-14C-antipyrine metabolism in man. Naunyn-Schmiedeberg's Arch Pharmacol 316:R-5
Böttcher J, Bässmann H, Schüppel R (1982a) Identification of sulfates in antipyrine metabolism in man, rat and rabbit. Drug Metab Dispos 10:90–91
Böttcher J, Bässmann H, Schüppel R (1982b) Quantitation and urinary pattern of 4,4′-dihydroxy-antipyrine, 4-hydroxy-antipyrine and 3-hydroxymethyl-antipyrine, as main metabolites of antipyrine in man and rat. J Pharm Pharmacol 34:168–175
Bremser W, Ernst L, Franke B, Gerhards R, Hardt A (1979) Carbon-13 NMR Spectral Data, Verlag Chemie, Weinheim
Cone EJ, Gorodetzky ChW (1975) Analytical controls in drug metabolic studies. A commentary. Drug Metab Dispos 3:417–418
Danhof M (1980) Antipyrine metabolite profile as a tool in the assessment of the activity of different drug oxidizing enzymes in man. Proefschrift Rijksuniversiteit Leiden, The Netherlands, p 189, Table 1
Danhof M, de Groot-van der Vis E, Breimer DD (1979) Assay of antipyrine and its primary metabolites in plasma, saliva and urine by high performance liquid chromatography and some preliminary results in man. Pharmacology 18:210–223
Deschamps J, Sauvaitre H, Arriau J, Maquestiau A, van Haverbeke Y, Jaquerye R (1971) Influence des solvants sur l'equilibre prototropique des pyrazoline-5-ones. Tetrahedron Lett 31:2929–2932
Dutton GJ (1980) Glucuronidation of drugs and other compounds. CRC Press, Boca Raton Fla
Eichelbaum M, Sonntag B, Dengler HJ (1981) HPLC determination of antipyrine metabolites. Pharmacology 23:192–202
Feeney J, Newman GA, Pauwels PJS (1970) A study of pyrazolin-5-onetautomerism IV. Natural abundance 13NMR spectra. J Chem Soc (C) 1970:1842–1844
Fenselau C, Johnson LP (1980) Analysis of intact glucuronides by mass spectrometry and gas chromatography-mass spectrometry. Drug Metab Dispos 8:274–283
Fishman WH (ed) (1970) Metabolic conjugation and hydrolysis vol I–III. Academic Press, New York, I, p 251
Hignite CE, Tschanz C, Huffman DH, Azarnoff DL (1978) Quantitation of N-demethylantipyrine in biological samples and isolation and characterization of its glucuronic acid conjugate. Drug Metab Dispos 6:288–295
Inaba T, Fischer E (1980) Antipyrine metabolism in man: simultaneous determination of norantipyrine and 4-hydroxy-antipyrine in urine by gas chromatography. Can J Physiol Pharmacol 58:17–21
Inaba T, Lucassen M, Kalow W (1980) Antipyrine metabolism in the rat by three hepatic monooxygenases. Life Sci 26:1977–1983
Inaba T, Uchino H, Kalow W (1981) Identification of p(4′)-hydroxyantipyrine as a metabolite of antipyrine in man. Res Commun Chem Pathol Pharmacol 33:3–8
Lehmann WD, Fischer R (1981) High temperature activated carbon emitters for field desorption mass spectrometry. Anal Chem 53:743–747
Mulder GJ (ed) (1981) Sulfation of drugs and related compounds, CRC Press, Boca Raton Fla
Schmid K, Künig W, Riess W, Dollery CT, Harland SJ (1981) Metabolism of hydralazine in man. Drug Res 31:1143–1147
Schüppel R (1966) Die Stickstoff-Demethylierung am Phenazon (Antipyrin). Naunyn-Schmiedeberg's Arch Exp Pathol Pharmacol 255:71–72
Schüppel R, Bässman H, Böttcher J (1981) Complete pattern of urinary metabolites of antipyrine in man — direct quantitation of conjugates, 8th Int Congr of Pharmacol, Abstract 1107, Tokyo
Schulten H-R (1979) Biochemical, medical, and environmental application of field-ionization and field desorption mass spectrometry. Int J Mass Spectrum Ion Phys 32:97–283
Zietz E, Eichelbaum M, Dengler HJ, Spiteller G (1978) Zum Metabolismus von Antipyrin (Phenazon) beim Menschen. Drug Res 28:315–319
Author information
Authors and Affiliations
Additional information
Supported by Deutsche Forschungsgemeinschaft, Bonn, FRG
Rights and permissions
About this article
Cite this article
Böttcher, J., Bässmann, H., Schüppel, R. et al. Preparative isolation and purification of urinary conjugates in antipyrine metabolism in man and rat. Naunyn-Schmiedeberg's Arch. Pharmacol. 321, 226–233 (1982). https://doi.org/10.1007/BF00505491
Issue Date:
DOI: https://doi.org/10.1007/BF00505491