Abstract
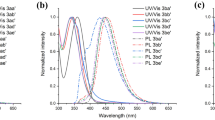
The condensation of hydrochloric salts of ω-aminomethyl aryl ketones with diphenic anhydride or with the diacid chloride of diphenic acid and subsequent cyclodehydration of the condensation products formed give sterically hindered mono- and bis(2, 5-diaryloxazoles) containing the biphenyl system. The spectroluminescent properties of these compounds were studied. Comparison of the absorption spectra of 2,2′-di(5-phenyloxazolyl-2)biphenyl and 2,5-diphenyloxazole indicates the complete lack of conjugation between the diphenyloxazole fragments, their independent behavior, and their retention of the spatial configuration of 2,5-diphenyloxazole. 2,2′-Di(5-phenyloxazolyl-2)biphenyl has a large Stokes shift. Steric hindrance is also found in 2-carboxy-2′-(5-phenyloxazolyl-2)biphenyl molecules.
Similar content being viewed by others
Literature cited
Luminescent Materials and Chemicals. NIITÉKhim Catalog [in Russian], Cherkassy (1975), p. 180.
G. A. Abakumov, V. V. Fadeev, R. V. Khokhlov, and A. P. Simonov, Spectrosc. Lett., 8, 651 (1975).
B. Williamson and W. Rodebush, J. Am. Chem. Soc., 63, 3018 (1941).
O. Bastiansen, Acta Chem. Scand., 3, 408 (1949); 4, 926 (1950).
L. M. Litvinenko and A. P. Grekov, Ukr. Khim. Zh., 20, 194 (1954).
A. G. London and R. Z. Mazengo, Org. Mass Spectrom., 8, 179 (1974).
E. D. Schmid and R. D. Tomsom, J. Am. Chem. Soc., 103, 1628 (1981).
G. H. Beaven and D. M. Hall, J. Chem. Soc., 4637 (1956).
B. M. Krasovitskii and D. G. Pereyaslova, Dokl. Akad. Nauk SSSR, 98, 71 (1957).
R. J. Morris and W. R. Brode, J. Am. Chem. Soc., 70, 2485 (1948).
W. R. Brode and R. J. Morris, J. Org. Chem., 13, 200 (1948).
B. M. Krasovitskii, V. B. Smelyakova, and R. N. Nurmukhametov, Opt. Spektrosk., 17, 558 (1964).
A. C. Littlejohn and J. W. Smith, J. Chem. Soc., 2552 (1954).
R. Roberts and T. Johnson, J. Am. Chem. Soc., 47, 1396 (1925).
C. Graebe and C. Aube, Ann., 247, 257 (1888).
F. Bell and P. H. Robinson, J. Chem. Soc., 1695 (1927).
Author information
Authors and Affiliations
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, No. 2, pp. 184–188, February, 1985.
Rights and permissions
About this article
Cite this article
Krasovitskii, B.M., Shapiro, S.G. & Yushko, É.G. Sterically hindered mono- and bis(2,5-diaryloxazoles) containing biphenyl systems. Chem Heterocycl Compd 21, 149–153 (1985). https://doi.org/10.1007/BF00504197
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00504197