Summary
-
1.
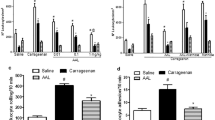
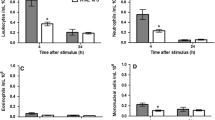
The influence of counter irritation by turpentine on carrageenan-oedema, leucocyte count, plasma kininogen stores and composition of sponge-induced exudates has been investigated in the rat.
-
2.
Counter irritation reduced the carrageenan-oedema in normal as well as in adrenalectomized rats. It induced leucopenia with lymphopenia but did not modify the plasma kininogen stores.
-
3.
In turpentine-pretreated rats, the exudates induced by sponge implantation 18 h previously had a lower content in leucocytes. Their levels in β-glucuronidase and β-galactosidase were slightly reduced, their content in PGE2 was not modified and their level in malonaldehyde was increased.
The exudates induced by sponge implantation 4 h previously had a lower content in leucocytes and PGE2 while their level in kinins was not modified.
-
4.
The mechanism of the anti-inflammatory effect of counter irritation by turpentine is discussed. We suggest that the main factor involved is a decrease in leucocyte accumulation into the exudates.
Similar content being viewed by others
References
Al-Haboubi HA, Zeitlin IJ (1983) Re-appraisal of the role of histamine in carrageenan-induced paw oedema. Eur J Pharmacol 88:169–176
Atkinson DC, Hicks R (1974) Possible role of adrenergic mechanisms in the systemic anti-inflammatory activity of acetic acid in rats. Eur J Pharmacol 26:158–165
Baldo BA (1982) Inflammation, counter irritation and serum acute phase α2-macroglobulin levels. Agents Actions 12:333–339
Bird J, Mohd-Hidir S, Lewis DA (1983) Putrescine — a potent endogenous anti-inflammatory substance in inflammatory exudates. Agents Actions 12:342–347
Bonta IL (1978) Endogenous modulators of the inflammatory response. Handb Exp Pharmacol 50/I:523–569
Borges DR, Gordon AH (1976) Kininogen and kininogenase synthesis by the liver of normal and injured rats. J Pharm Pharmacol 28:44–48
Bragt PC, Bonta IL (1979) “In vivo” metabolism of (1-14C) arachidonic acid during different phases of granuloma development in the rat. Biochem Pharmacol 28:1581–1586
Bragt PC, Bonta IL (1979) Metabolism of arachidonate “in vivo” during different phases of granuloma development in the rat. In: Weissman G, Samuelsson B, Paoletti R (eds) Advances in inflammation research. vol I, Raven Press, New York, pp 499–502
Bragt PC, Schenkelaars EPM, Bonta IL (1979) Dissociation between prostaglandin and malonaldehyde formation in exudate and increased levels of malonaldehyde in plasma and liver during granulomatous inflammation in the rat. Prostagl Med 2:51–61
Damas J (1982) Contribution à l'étude du pouvoir inflammatoire des carragénines et de sa signification en tant que modèle physiopathologique. Thesis. University of Liège, Belgium
Damas J, Remacle-Volon G (1981) Rôle des kinines dans l'oedème tégumentaire déclenché par les carragénines chez le rat. J Pharmacol (Paris) 13:225–239
Damas J, Bourdon V, Lecomte J, Salmon J (1982) Plaquettes, enzymes lysosomiales et choc anaphylactiques chez le rat. CR Soc Biol 176:553–557
Damas J, Juchmes-Ferir A, Volon G (1978) Les actions hypotensives et oedématogènes de la carragénine iota chez le rat irradié. C R Soc Biol 172:377–378
Deflandre E, Damas J, Adam A (1983) Prostaglandin biosynthesis is not affected by the anti-inflammatory effect of turpentine. Prostaglandins Leuko Med 12:179–188
Diniz CR, Carvalho IF (1963) A micromethod for determination of bradikininogen under several conditions. Ann NY Acad Sci 104:77–82
Di Rosa M, Giroud JP, Willoughby DA (1971) Studies of the mediators of the acute inflammatory response induced in rats in different sites by carrageenan and turpentine. J Pathol 104:15–29
Doyle AB, Hambleton P, Miller P, Parker F (1983) Modulation of cell accumulation and inflammatory enzyme release in implanted sponges. Br J Pharmacol 79:350P
Ford-Hutchinson AW, Walker JR, Smith MJH (1978) Assessment of anti-inflammatory activity by sponge implantation techniques. J Pharmacol Meth 1:3–7
Garcia Leme J, Schapoval EES (1975) Stimulation of the hypothalamopituitary-adrenal axis by compounds formed in inflamed tissue. Br J Pharmacol 53:75–83
Glenn EM, Bowman BJ, Koslowske TC (1968) The systemic response to inflammation. Biochem Pharmacol, suppl 27–49
Higgs GA (1984) Effects of anti-inflammatory drugs on arachidonic acid metabolism and leukocyte migration. In: Otterness I, Capetola R, Wong S (eds) Advances in inflammation research. vol 7, Raven Press, New York, pp 223–238
Higgs GA, Moncada S, Salmon JA, Seager K (1983) The source of thromboxane and prostaglandins in experimental inflammation. Br J Pharmacol 79:863–868
Kushner I (1982) The phenomenom of the acute phase response. Ann NY Acad Sci 389:39–48
Roch-Arveiller M, Bradshaw D, Giroud JP (1979) Relationship between inhibitors of rat polymorphonuclear chemotaxis and various inflammatory reactions. Agents Actions 9:289–293
Saxena PN (1960) Effects of drugs on early inflammatory reaction. Arch Int Pharmacodyn Ther 126:228–237
Simmons PM, Salmon JA, Moncada S (1983) The release of leukotriene B4 during experimental inflammation. Biochem Pharmacol 32:1353–1359
Simonart A (1967) Pharmacodynamie et thérapeutique. Arscia Maloine, Bruxelles Paris, pp 859–865
Suggio K, Dali JW (1983) Effect of forskolin on alterations of vascular permeability induced with bradykinin, prostaglandin E1, adenosine, histamine and carrageenin in rats. Life Scienc 33:65–73
Timsit J, Aminot A, Lechat P (1978) The influence of anti-inflammatory compounds on some effects of BCG in the rat. Arch Int Pharmacodyn Ther 235:271–279
Trautschold I (1970) Assay methods in the kinin system. Handb Exp Pharmacol 25:52–81
Ufkes JGR, Zeegers A, Boers W, Van Gool J (1983) Inhibitory effect of BaSO4 on experimentally induced inflammation in the rat mediated by αM-foetoprotein. Arch Int Pharmacodyn Ther 262:287–298
Van Arman CG (1979) Oedema and increased vascular permeability. Handb Exp Pharmacol 50/II:75–91
Weissmann G, Serhan C, Korchak HM, Smolen JE (1982) Neutrophils: Release of mediators of inflammation with special reference to rheumatoid arthritis. Ann NY Acad Sci 389:11–24
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Damas, J., Deflandre, E. The mechanism of the anti-inflammatory effect of turpentine in the rat. Naunyn-Schmiedeberg's Arch. Pharmacol. 327, 143–147 (1984). https://doi.org/10.1007/BF00500909
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00500909