Summary
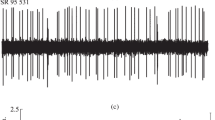
Dextro-and levorotatory isomers of 1-(1-phenylcyclohexyl)-3-methylpiperidine (PCMP) were synthesized. Both isomers inhibited spontaneous cerebellar Purkinje neuron firing when applied locally by pressure ejection. This effect was dose-dependent, with the (+)-isomer about 5–7 times more potent than the (−)-isomer. Both isomers also depressed rotarod performance in mice. Again, the (+)-isomer was about 5 times more potent than the (−)-isomer. Both rotarod performance and Purkinje cell discharge were depressed maximally 10–15 min after i.p. injection of drug. Our results suggest a correlation between behavioral performance and central neuron electrophysiological activity and suggest that the central actions of PCP or its derivatives are probably mediated at one locus, by a stereospecific mechanism.
Similar content being viewed by others
References
Adams PM (1980) Interaction of phencylidine with drugs affecting cholinergic transmission. Neuropharmacology 19:151–153
Betoni G, Perrone R, Tortorella V (1972) Absolute configuration and optical purity of 3-methylpiperidine. Gaz Chim Ital 102:196–204
Bloom FE (1978) Central noradrenergic systems: Physiology and pharmacology. In: Lipton ME, Killam KC, DiMascio A (eds) Psychopharmacology — A Generation of Progress. Raven Press New York, pp 131–149
Bloom FE, Algeri S, Groppetti A, Revuelta A, Costa E (1969) Lesions of central adrenergic terminals with 6-hydroxydopamine: Biochemistry and fine structure. Science 166:1284–1286
Dale JA, Dull DL, Mosher HS (1969) α-Methoxy-α-trifluoromethylphenylacetic acid, a versatile reagent for the determination of enantiomeric composition of alcohols and amines. J Org Chem 34:2543–2549
Davidson W (1976) Neurotransmitter amino acids. Academic Press New York
Dunham NW, Miya TS (1956) A note on a simple apparatus for detecting neurologic deficit in rats and mice. J Am Pharm Assoc 3:112–113
Eccles JC, Ito M, Szentogathai J (1967) The Cerebellum as a Neuronal Machine. Springer New York
Fessler RG, Sturgeon RD, Meltzer HY (1979) Phencyclidine-induced rotation in rats with unilateral 6-hydroxydopamine-induced lesions of the substantia nigra. Life Sci 24:1281–1288
Freedman R, Hoffer BJ (1975) Phenothiazine antagonism of the noradrenergic inhibition of cerebellar Purkinje neurons. J Neurobiol 6:277–288
Freedman R, Hoffer BJ, Woodward DJ (1975) A quantitative microiontophoretic analysis of the responses of central neurones to noradrenaline. Br J Pharmacol 54:529–539
Glick SD, Cox RD, Maayani S, Meibach RC (1979) Anticholinergic behavioral effect of phencyclidine. Eur J Pharmacol 59:103–106
Hoffer BJ, Siggins GR, Oliver AP, Bloom FE (1973) Activation of the pathway from locus coeruleus to rat cerebellar Purkinje neurons: Pharmacological evidence of noradrenergic central inhibition. J Pharmacol Exp Ther 184:553–569
Maayani S, Weinstein H (1980) Specific binding of [3H] phencyclidine: Artifacts of the rapid filtration method. Life Sci 26:2011–2022
Maddox VH, Godefroi EF, Parcell RF (1965) The synthesis of phencyclidine and other 1-arylcyclohexylamines. J Med Chem 8:230–235
Marwaha J, Palmer M, Hoffer B, Freedman R (1980a) Phencylidine induced depressions of cerebellar Purkinje neurons. Life Sci 26:1509–1516
Marwaha J, Palmer M, Woodward DJ, Hoffer BJ, Freedman R (1980b in press) Electrophysiological evidence for presynaptic actions of phencyclidine on noradrenergic terminals in rat cerebellum. J Pharmacol Exp Ther
Masamune T, Takasugi M, Murai A (1971) The synthesis of veratramine. Tetrahedron 27:3369–3386
McCaman RE, McKenna DG, Ono JK (1977) A pressure system for intracellular and extracellular ejections of picoliter volumes. Brain Res 136:141–147
Mohler H, Okada T (1977) Benzodiazepine receptor: Demonstration in the central nervous system. Science 198:849–851
Newman P (1977) Optical resolution procedures for chemical compounds, Vol 1. Optical Resolution Information Center, Manhattan College, Riverdale, New York p 588–590
Nicoll RA, Siggins GR, Ling N, Bloom FE, Guillemin R (1977) Neuronal actions of endorphins and enkephalins among brain regions: A comparative microiontophoretic study. Proc Natl Acad Sci USA 74:2584–2589
Palmer MR, Hofer BJ (1980) Catecholamine modulation of enkephalin-induced electrophysiological responses in cerebral cortex. J Pharmacol Exp Ther 213:205–215
Palmer MR, Wuerthele SM, Hoffer BJ (1980) Physical and physiological characteristics of micropressure ejection of drugs from multibarreled pipettes. Neuropharmacology 19:931–938
Raja SN, Guyenet PG (1980) Effects of phencyclidine on the spontaneous activity of monoaminergic neurons. Eur J Pharmacol 62:229–233
Sakai M, Swartz BE, Woody CD (1979) Controlled micro-release of pharmacological agents: Measurement of volumes ejected in vitro through fine tipped glass microelectrodes by pressure. Neuropharmacology 18:209–213
Siggins GR, Henriksen SJ, Bloom FE (1979) Iontophoresis of Li+ antagonizes noradrenergic synaptic inhibition of rat cerebellar Purkinje cells. Proc Natl Acad Sci USA 76:3015–3018
Simon EJ (1976) The opiate receptors. Neurochem Res 13:28–41
Smith RC, Meltzer HY, Dekirmenjian H, Davis JM (1975) Effects of phencyclidine on biogenic amines in rat brain. Neuroscience Abstracts 1
Sorensen S, Palmer M, Dunwiddie T, Hoffer B (1980) Electrophysiological correlates of ethanol-induced sedation in differentially sensitive lines of mice. Science 210:1143–1145
Vincent JP, Kartalovski B, Geneste P, Kamenka JM, Lazdunski M (1979) Interaction of phencyclidine (“angel dust”) with a specific receptor in rat brain membranes. Proc Natl Acad Sci USA 76:4678–4682
Zukin SR, Zukin S (1979) Specific [3H]phencyclidine binding in rat central nervous system. Proc Natl Acad Sci USA 76:5372–5376
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Marwaha, J., Palmer, M., Hoffer, B. et al. Differential electrophysiological and behavioral responses to optically active derivatives of phencyclidine. Naunyn-Schmiedeberg's Arch. Pharmacol. 315, 203–209 (1981). https://doi.org/10.1007/BF00499836
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00499836