Summary
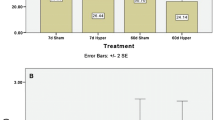
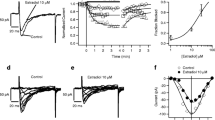
Experiments were carried out to investigate the nature of the calcium homeostatic mechanisms in neoplastic GH3 rat pituitary cells. GH3 cells grown and maintained in Ham's F10 culture medium containd 35 nmoles calcium/mg cell protein. When stimulated by thyrotropin releasing hormone (TRH) or elevated K+ concentrations, only the latter caused cell calcium levels to rise although both resulted in hormone release. Then exposed to EGTA, the GH3 cells lost calcium. When the temperature was lowered to 4°C, the cells gained calcium and when rewarmed were able to extrude the previously accumulated calcium. The increased cell calcium following cold exposure could be blocked by prior treatment with rotenone. If rotenone was added subsequent to the cold exposure, it did not block the extrusion seen upon rewarming. In the absence of glucose in the medium, the GH3 cells took up more calcium upon exposure to 4° C, and upon rewarming the cells could not return to their previous low levels. There are thus significant differences in calcium homeostasis between the neoplastic GH3 cells and their normal pituitary counterparts. When intracellular calcium was localized with the potassium pyroantimonate technique, there was calcium found in/on mitochondria, membrane bound vesicles and plasma membrane. Nuclear staining was sparse, and nucleolar staining was virtually absent. Upon stimulation with TRH, there was a decrease in mitochondrial calcium along with increases in both plasma membrane and nucleolar calcium levels. Since total calcium is unchanged, this indicates a significant calcium redistribution in response to TRH. The increased nucleolar calcium may reflect a calcium dependent increase in mRNA synthesis as has been reported. Since TRH presumably acts at a surface receptor, the increased plasma membrane calcium might be functionally related to receptor activation.
Similar content being viewed by others
References
Borowitz JL (1969) Effect of acetylcholine on the subcellular distribution of 45C2+ in bovine adrenal medulla. Biochem Pharmacol 18:715–723
Cittadini A, Bossi D, Rosi G, Wolf F, Terranova T (1977) Calcium metabolism in Ehrlich ascites tumour cells. Biochim Biophys Acta 469:345–349
Clemente F, Meldolesi J (1975a) Calcium and pancreatic secretion. I. Subcellular distribution of calcium and magnesium in the exocrine pancreas of the guinea pig. J Cell Biol 65:88–102
Clemente F, Meldolesi J (1975b) Calcium and pancreatic secretion — dynamics of subcellular calcium pools in resting and stimulated acinar cells. Br J Pharmacol 55:368–379
Cramer EB, Cardassis C, Pereira G, Milks L, Ford D (1978) Ultrastructural localization of cations in the rat pars distalis under various experimental conditions. Neuroendocrinology 26:72–84
Dormer RL, Poulsen JH, Licko V, Williams JA (1981) Calcium fluxes in isolated pancreaticacini: effects of secretagogues. Am J Physiol 240:G38-G49
Dormer RL, Williams JA (1981) Secretagogue-induced changes in subcellular Ca2+ distribution in isolated pancreatic acini. Am J Physiol 240:G130-G140
Eto S, Wood JM, Hutchins M, Fleischer N (1974) Pituitary 45Ca++ uptake and release of ACTH, GH and TSH: Effect of verapamil. Am J Physiol 226:1315–1320
Gautvik KM, Tashjian AM Jr (1973a) Effects of Ca++ and Mg++ on secretion and synthesis of growth hormone and prolactin by clonal strains pituitary cells in culture. Endocrinology 92:573–583
Gautvik KM, Tashjian AM Jr (1973b) Effects of cations and colchicine on the release of prolactin and growth hormoen by functional pituitary tumor cells in culture. Endocrinology 93:793–799
Gershengorn MC (1980) Thyrotropin releasing hormone stimulation of prolactin release. Evidence for a membrane potential independent, Ca2+-dependent mechanism of action. J Biol Chem 255:1980–1803
Landry Y, Lehninger AL (1976) Transport of calcium ion by Ehrlich ascites tumor cells. Biochem J 158:427–438
Leuschen MP, Moriarty CM, Sampson HW, Piscopo I (1981) Cytochemicalanalysis of intracellular calcium distribution in the rat anterior pituitary. Cell Tissue Res 220:191–200
Milligan JV, Kraicer J (1979) The effects of prolonged chilling upon in vitro Ca2+ accumulation, influx and growth hormone release in rat adenohypophysis. Can J Physiol Pharmacol 57:1359–1364
Moriarty CM (1980) Kinetic analysis of calcium distribution in rat anterior pituitary slices. Am J Physiol 238:E167-E173
Moriarty CM, Leuschen MP (1981) Role of calcium in acute stimulated release of prolactin from neoplastic GH3 cells. Am J Physiol 240:E705-E711
Moriarty CM, Leuschen MP, Campbell GT (1977) Variations in the response of enzyme-dissociated rat pituitary cells to thyrotropin releasing hormone. Life Sci 23:2073–2078
Ostlund RE Jr, Leung JT, Hajek SV, Winokur T, Melman M (1978) Acute stimulated hormone release from cultured GH3 pituitary cells. Endocrinology 103:1245–1252
Schecter J (1976) Cations in the rat pars distalis ultrastructural localization. Am J Anat 146:189–206
Stoeckel ME, Hindeland-Gertner C, Dellman HD, Park A, Stutinsky F (1975) Subcellular localization of calcium in the mouse hypophysis. Cell Tissue Res 157:307–322
Tashjian AH Jr, Lomedico ME, Maina D (1978) Role of calcium in the thyrotropin-releasing hormone-stimulated release of prolactin from pituitary cells in culture. Biochem Biophys Res Commun 81:798–806
White BA, Baverle LR, Bancroft FC (1981) Calcium specifically stimulates prolactin synthesis and messenger RNA sequences in GH3 cells. J Biol Chem 256:5942–5945
Yancey SB, Weiner R, Schecter J (1980) Calcium and the secretory cycle of prolactin cells: A cytochemical and ultrastructural study of dopamine inhibition and monobutyiyl cyclic AMP stimulation of prolactin secretion. Am J Anat 157:345–356
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Leuschen, M.P., Moriarty, C.M. & Sampson, H.W. Calcium movements and intracellular calcium distribution in neoplastic GH3 cells. Histochemistry 77, 85–97 (1983). https://doi.org/10.1007/BF00496639
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00496639