Abstract
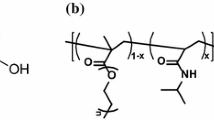
The synthesis of transition metal based hybrid copolymers is achieved by using transition metal alkoxides modified by chelating ligands functionalized with polymerizable organic groups. The heterofunctional precursor is an acetoacetoxyethylmethacrylate modified zirconium propoxyde. The hybrid copolymers obtained by double polymerization of heterofunctional precursors are characterized in the liquid and in the solid state by using light scattering, SAXS measurements, UV-visible, FTIR, 13C MAS NMR spectroscopies and several chemical and gravimetric analyses. Both inorganic polycondensation and organic polymerization occured and the chemical bond between organic and inorganic moities is conserved. These hybrids consist of polyzirconates chemically bonded to polymeric methacrylate chains via the β-diketo complexing function. The determination of the conversion degree of both polymerization reactions reveals the competition between the two types of reactions. This competition controls the scale of homogeneity. The modification ratio (R = AAEM/Zr) of zirconium alkoxide appears to be the key parameter for the tuning of the homogeneity. A careful adjustment of this parameter leads to zirconium oxo species with more or less open structures and to the tailoring of the ratio between organic and inorganic components.
Similar content being viewed by others
References
C.J. Brinker and G. Scherrer, Sol-Gel Science, the Physics and Chemistry of Sol-Gel Processing (Academic press, San-Diego, 1989).
Sol-Gel Technology for Thin Films, Fiber, Preforms, Electronics and Especialty Shapes, edited by L. C. Klien (Noyes Pub., 1988).
J. Livage, M. Henry, and C. Sanchez, Progress in Solid State Chemistry 18, 259 (1988).
C.D. Chandler, C. Roger, and M. Hampden-Smith, Chem. Rev. 93, 1205 (1993).
Better Ceramics Through Chemistry I to VII, Mat. Res. Soc. Symp. Proc. 73 (1986). 121 (1988), 180 (1990), 271 (1992), 346 (1994).
D. Avnir, D. Levy, and R. Reisfeld, J. Phys. Chem. 88, 5956 (1984).
H. Schmidt and B. Seiferling, Mat. Res. Soc. Symp. Proc. 73, 739 (1986).
G.L. Wilkes, B. Orler, and H.H. Huang, Polymer Prep. 26(2), 300 (1986).
G.-S. Sur and J.E. Mark, Eur. Polym. J. 21(12), 1051 (1986).
a) Sol-Gel Optics I, edited by J.D. Mackenzie and D.R. Ulrich (Proc. SPIE 1328, Washington, 1990).
b) Sol-Gel Optics II, edited by J.D. Mackenzie (Proc. SPIE 1758, Washington, 1992).
c) Sol-Gel Optics III, edited by J.D. Mackenzie (Proc. SPIE 2288, Washington, 1994).
Sol-Gel Optics, Processing and Applications, edited by L.C. Klein (Kluwer Academic Publishers, Boston, 1993).
H.H. Huang, B. Orler, and G.L. Wilkes, Macromolecules 20, 1322 (1987).
B.M. Novak, Adv. Mater. 5(6), 422 (1993).
Y. Chujo and T. Saegusa, Advances in Polymer Science 100, 11 (1992).
B.K. Coltrain, C.J.T. Landry, J.M. O'Reilly, A.M. Chamberlain, G.A. Rakes, J.S.S. Sedita, L.W. Kelts, M.R. Landry, and V.K. Long, Chem. Mater. 5, 1445 (1993).
C. Sanchez and F. Ribot, New Journal Chemistry 18(10), 1007 (1994).
A. Morikawa, Y. Iyoku, M. Kakimoto, and Y. Imai, J. Mater. Chem. 2(7), 679 (1992).
K.J. Shea, D.A. Loy, and O. Webster, J. Am. Chem. Soc. 114, 6700 (1992).
R.J.P. Corriu, J.J.E. Moreau, P. Thepot, and M. Wong Chi Man, Chem. Mater. 4, 1217 (1992).
F. Ribot, F. Banse, and C. Sanchez, Mat. Res. Soc. Symp. Proc. 346, 121 (1994).
F. Ribot, F. Banse, F. Diter, and C. Sanchez, New Journal Chemistry 19, 10, 1163 (1995).
F. Banse, F. Ribot, C. Sanchez, M. Lahcini, and B. Jouseaume, JSST (submitted).
J.C. Debsikar, J. Non-Cryst. Solids 87, 343 (1986).
W.C. Lacourse and S. Kim in Science of Ceramic Processing, edited by L.L. Hench and D.R. Ulrich (Wiley, New-York, 1986), p. 304.
H. Unuma, T. Tokoda, T.Y. Susuki, T. Furusaki, K. Kodaira, and T. Hatsushida, J. Mater. Sci. Lett. 5, 1248 (1986).
P. Papet, N. LeBars, J.F. Baumard, A. Lecomte, and A. Dauger, J. Mater. Sci. 24, 3850 (1989).
C. Sanchez, J. Livage, M. Henry, and F. Babonneau, J. Non-Cryst. Solids 100, 650 (1988).
A. Leaustic, F. Babonneau, and J. Livage, Chem. Mater. 1, 248 (1989).
F. Ribot, P. Toledano, and C. Sanchez, Chem. Mater. 3, 759 (1991).
M. In (PHD 1994 Martin In University of Paris VI, France).
A.L. Suvorov and S.S. Spasskii, Proc. Acad. Sci. USSR 127, 615 (1959).
R. NaB and H. Schmidt in Sol-Gel Optics I, edited by J.D. Mackenzie and D.R. Ulrich (Proc. SPIE 1328, Washington, 1990), p. 258.
U. Schubert, E. Arpac, W. Glaubitt, A. Helmerich, and C. Chau, Chem. Mater. 3, 291 (1992).
C. Sanchez and M. In, J. Non-Cryst. Solids 147 & 148, 1 (1992).
E. Tsushida, Nishide, Adv. Polym. Sci. 24, 1 (1977).
J. Lambard and T. Zemb, J. de Physique 2, 1191 (1992).
P. Toledano, M. In, and C. Sanchez, C. R. Acad. Sci. Paris Serie II 313, 1247 (1991).
T.A. Ulibarri, G. Beaucage, D.W. Schaefer, B.J. Oliver, and R.A. Assink, Mater. Res. Soc. Proc. Symp. 274, 85 (1992).
M.R. Landry, B.K. Coltrain, C.J.T. Landry, and J.M. O'Reilly, J. Polymer. Science B: Polymer Physics 33, 637 (1995).
C.S. Betratet and G.L. Wilkes, Chem. Mater. 7, 535 (1995).
P.G. de Gennes, J. Physique Lett. 40, L-69 (1979).
J. Blanchard, S. Doeuff-Barboux, J. Maquet, and C. Sanchez, New Journal Chemistry 19, 8 and 9, 929 (1995).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
In, M., Gérardin, C., Lambard, J. et al. Transition metal based hybrid organic-inorganic copolymers. J Sol-Gel Sci Technol 5, 101–114 (1995). https://doi.org/10.1007/BF00487726
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00487726