Abstract
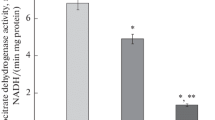
Genetic analyses, involving backcross and F2 matings, demonstrate that the type I hyperprolinemia of PRO/Re mice is caused by an abnormal allele at a single locus designated pro-1. Mice homozygous for this allele (pro-1b/pro-1b) possess a deficiency in the activity of component 1 of mitochondrial proline dehydrogenase. In liver mitochondria of normal C57BL/6J mice, two proline dehydrogenase activity components are demonstrable by electrophoretic resolution of Triton X-100 solubilized extracts. In mitochondria of PRO/Re mice, the activity of component 1 is not readily detectable. Residual proline dehydrogenase activity in PRO/Re mitochondria appears, therefore, to be due in large measure to activity component 2 which is more stable to incubation at 40 C, exhibits slower electrophoretic mobility, and is less reactive to menadione. Kinetic analyses demonstrate a K m (proline) for the Triton X-100 solubilized enzyme activities of PRO/Re and C57BL/6J liver mitochondria of 0.4 M and 2.9×10−3 M, respectively. C57BL/6J enzyme activity is inhibited by high substrate concentration. The activity of component 1 was not detected in other subcellular fractions of PRO/Re liver obtained by differential centrifugation. Abnormal control of respiratory chain function in PRO/Re mitochondria appears to involve primarily proline oxidation, as indicated by the level of activity of several inner membrane enzymes.
Similar content being viewed by others
References
Blake, R. L. (1972). Animal model for hyperprolinemia: Deficiency of mouse proline oxidase activity. Biochem. J. 129987.
Blake, R. L., and Russell, E. S. (1972). Hyperprolinemia and prolinuria in a new inbred strain of mice, PRO/Re. Science 176809.
Brosemer, R. W., and Veerabhadrappa, P. S. (1965). Pathway of proline oxidation in insect flight muscle. Biochim. Biophys. Acta 110102.
Brunner, G., and Neupert, W. (1969). Localization of proline oxidase and pyrroline 5-carboxylic acid dehydrogenase in rat liver. FEBS Letters 3283.
Buchler Instruments (1971). Instructions for the Polyanalyst: An Analytical Temperature-Regulated Electrophoresis Apparatus, Buchler Instruments Division, Fort Lee, N.J.
Efron, M. L. (1965). Familial hyperprolinemia: Report of a second case, associated with congenital renal malformations, hereditary hematuria and mild mental retardation, with demonstration of an enzyme defect. New Engl. J. Med. 2721243.
Erecinska, M. (1966). Ubiquinone in proline oxidation. Acta Biochim. Polon. 13209.
Fleischer, S., and Fleischer, B. (1967). [70] Removal and binding of polar lipids in mitochondria and other membrane systems. D. Assay of β-hydroxybutyric dehydrogenase activity. In Estabrook, R. W., and Pullman, M. E. (eds.), Methods in Enzymology, Vol. X, Academic Press, New York, p. 429.
Fleischer, S., and Kervina, M. (1974). [2] Subcellular fractionation of rat liver. In Fleischer, S., and Packer, L. (eds.), Methods in Enzymology, Vol. XXXIA, Academic Press, New York, p. 6.
Gardner, R. S. (1974). A sensitive colorimetric assay for mitochondrial α-glycerophosphate dehydrogenase. Anal. Biochem. 59272.
Johnson, A. B., and Strecker, H. J. (1962). The interconversion of glutamic acid and proline. IV. The oxidation of proline by rat liver mitochondria. J. Biol. Chem. 2371876.
Kramar, R. (1967). Studies on the proline oxidase complex. Enzymology 3333.
Lowry, O. H., Rosebrough, N. J., Farr, A. L., and Randall, R. J. (1951). Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193265.
Peraino, C., and Pitot, H. C. (1962). Proline synthesis and degradation in a minimal deviation hepatoma. Biochim. Biophys. Acta 62585.
Rothschild, H. A., Cori, O., and Barron, E. S. (1954). The components of choline oxidase and aerobic phosphorylation coupled with choline oxidation. J. Biol. Chem. 20841.
Scriver, C. R., and Efron, M. L. (1972). Disorders of proline and hydroxyproline metabolism. In Stanbury, J. B., Wyngaarden, J. B., and Frederickson, D. S. (eds.), The Metabolic Basis of Inherited Disease, McGraw-Hill, New York, p. 351.
Scriver, C. R., Schaefer, I. A., and Efron, M. L. (1961). A new renal tubular amino acid transport system and a new hereditary disorder of amino acid metabolism. Nature 192672.
Strecker, H. J. (1971). [174a] The preparation of animal proline oxidase (rat liver), and its use for the preparation of Δ1-pyrroline-5-carboxylate. In Tabor, H., and Tabor, C. W. (eds.), Methods in Enzymology, Vol. XVII, Academic Press, New York, p. 251.
Tisdale, H. D. (1967). [39] Preparation and properties of succinic-cytochrome c reductase (complex II–III). In Estabrook, R. W., and Pullman, M. E. (eds.), Methods in Enzymology, Vol. X, Academic Press, New York, p. 213.
Wharton, D. C., and Tzagoloff, A. (1967). [45] Cytochrome oxidase from beef heart mitochondria. In Estabrook, R. W., and Pullman, M. E. (eds.), Methods in Enzymology, Vol. X, Academic Press, New York, p. 245.
Author information
Authors and Affiliations
Additional information
This investigation was supported by NIH Research Grants AM 14769 from the National Institute of Arthritis, Metabolic, and Digestive Diseases and CA 01074 from the National Cancer Institute, and by an allocation from NIH General Research Support Grant RR 05545 from the Division of Research Resources to the Jackson Laboratory. The Jackson Laboratory is fully accredited by the American Association of Laboratory Animal Care.
Rights and permissions
About this article
Cite this article
Blake, R.L., Hall, J.G. & Russell, E.S. Mitochondrial proline dehydrogenase deficiency in hyperprolinemic PRO/Re mice: Genetic and enzymatic analyses. Biochem Genet 14, 739–757 (1976). https://doi.org/10.1007/BF00485338
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00485338