Abstract
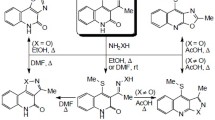
The reduction of the oximes of 2,2-dimethyl-4-chromanone (I), 2,2, 6-trimethyl-4-chromanone(II), and 2,2, 5, 7, 8-pentamethyl-6-hydroxy-4-chromanone (III) with lithium aluminum hydride has been investigated. The first of these affords the normal reduction product 2,2-dimethyl-4-amino chroman (VI), which was also obtained by catalytic hydrogenation of the oxime. The second oxime is converted into a mixture containing the 4-aminochroman (VII) and the product of reductive ring expansion, 2,2,7-trimethyl-2,3,4,5-tetrahydro-1, 5-benzoxazepine (VIII). From the oxime of III was obtained 2,2,6,8, 9-pentamethyl-7-hydroxy-2,3,4,5-tetrahydro-1,5-benzoxazepine (IX), the structure of which was confirmed by determination of the basicity constants, the NMR spectra, and the preparation of the N-benzoyl derivatives (XI).
Similar content being viewed by others
References
V. A. Zagorevskii and N. V. Dubykina, ZhOKh, 34, 2282, 1964.
N. V. Dubykina and V. A. Zagorevskii, The Synthesis of Natural Compounds and of Their Analogs and Fragments [in Russian], Nauka, 134, 1965.
W. John, Ph. Gunter, and M. Schmiel, Ber., 71, 2637, 1938.
H. Offe and W. Barkow, Ber., 80, 464, 1947.
W. Backer et al., J. Chem. Soc., 2010, 1956.
K. Auwers, Ann., 421, 1, 1920.
O. Emerson et al., J. Biol. Chem., 122, 99, 1937.
D. Huckle, I. Lockhart, and M. Wright, J. Chem. Soc., 1137, 1965.
D. Evans and I. Lockhart, J. Chem. Soc., 4806, 1965.
D. Lansburg and N. Mancuso, Tetrah. Let., 2445, 1965.
K. Lyle and H. Troscianiec, J. Org. Chem., 20, 1757, 1955.
M. Harfenist and E. Magien, J. Am. Chem. Soc., 80, 6080, 1958.
N. I. Kudryashova and N. V. Khromov-Borisov, ZhOrKh, 2, 578, 1966.
E. K. Orlova, I. D. Tsvetkova, V. S. Troitskaya, V. G. Vinokurov, and V. A. Zagorevskii, KhGS [Chemistry of Heterocyclic Compounds], 429, 1969.
Author information
Authors and Affiliations
Additional information
For part XXX, see [14].