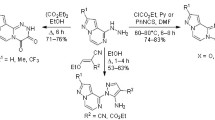
Abstract
A series of substituted pyridine-2-carbaldehydes were brought into heterocyclization with isonitrosoacetophenone hydrazones, followed by aromatization by the action of oxidants or by dehydration in boiling acetic acid. As a result, substituted 3-(pyridin-2-yl)-1,2,4-triazines or 3-(pyridin-2-yl)-1,2,4-triazine 4-oxides were formed. 6-Formylpyridine-2-carbonitrile failed to undergo heterocyclization, 6-methylpyridine-2-carbaldehyde and methyl 6-formylpyridine-3-carboxylate can be converted to both 1,2,4-triazine and 1,2,4-triazine 4-oxide derivative, and only 1,2,4-triazine 4 oxides were obtained from 6-bromopyridine-2-carbaldehyde and 6-formyl-3-phenylpyridine-2-carbonitrile. Convenient procedures were proposed for the synthesis of some initial pyridinecarbaldehydes.
Similar content being viewed by others
References
Kozhevnikov, V.N., Shabunina, O.V., Kopchuk, D.S., Ustinova, M.M., Köenig, B., and Kozhevnikov, D.N., Tetrahedron, 2008, vol. 64, p. 8963.
Kadoma, H., Kawakami, S., Nomura, H., Ushikubo, T., and Seo, S., US Patent no. 2012/178933 A1, 2012.
Pfueller, O.C. and Sauer, J., Tetrahedron Lett., 1998, vol. 39, p. 8821.
Catozzi, N., Bromley, W.J., Wasnaire, P., Gibson, M, and Taylor, R.J.K., Synlett, 2007, no. 14, p. 2217.
Kopchuk, D.S., Chepchugov, N.V., Kim, G.A., Zyryanov, G.V., Kovalev, I.S., Rusinov, V.L., and Chupakhin, O.N., Russ. Chem. Bull., Int. Ed., 2015, vol. 64, p. 695.
Altuna-Urquijo, M., Gehre, A., Stanforth, S.P., and Tarbit, B., Tetrahedron, 2009, vol. 65, p. 975.
Kopchuk, D.S., Nikonov, I.L., Zyryanov, G.V., Kovalev, I.S., Rusinov, V.L., and Chupakhin, O.N., Chem. Heterocycl. Compd., 2014, vol. 50, p. 907.
Kopchuk, D.S., Khasanov, A.F., Kovalev, I.S., Kim, G.A., Nikonov, I.L., Zyryanov, G.V., Rusinov, V.L., and Chupakhin, O.N., Chem. Heterocycl. Compd., 2014, vol. 50, p. 864.
Nikonov, I.L., Kopchuk, D.S., Kovalev, I.S., Zyryanov, G.V., Khasanov, A.F., Slepukhin, P.A., Rusinov, V.L., and Chupakhin, O.N., Tetrahedron Lett., 2013, vol. 54, p. 6427.
Kopchuk, D.S., Nikonov, I.L., Zyryanov, G.V., Nosova, E.V., Kovalev, I.S., Slepukhin, P.A., Rusinov, V.L., and Chupakhin, O.N., Mendeleev Commun., 2015, vol. 25, p. 13.
Kopchuk, D.S., Nikonov, I.L., Zyryanov, G.V., Kovalev, I.S., Taniya, O.S., Rusinov, V.L., and Chupakhin, O.N., Russ. J. Org. Chem., 2015, vol. 51, p. 1170.
Prokhorov, A.M., Kozhevnikov, D.N., Rusinov, V.L., Chupakhin, O.N., Glukhov, I.V., Antipin, M.Yu., Kazheva, O.N., Chekhlov, A.N., and Dyachenko, O.A., Organometallics, 2006, vol. 25, p. 2972.
Kozhevnikov, D.N., Kozhevnikov, V.N., Prokhorov, A.M., Ustinova, M.M., Rusinov, V.L., Chupakhin, O.N., Aleksandrov, G.G., and Köenig, B., Tetrahedron Lett., 2006, vol. 47, p. 869.
Kovalev, I.S., Kopchuk, D.S., Khasanov, A.F., Zyryanov, G.V., Rusinov, V.L., and Chupakhin, O.N., Mendeleev Commun., 2014, vol. 24, p. 117.
Dawson, M.V and Lyle, S.J., Talanta, 1990, vol. 37, p. 1189.
Ueno, Y., Morishita, K., Muraoka, M., and Ohashi, N., US Patent no. 6 159 974, 2000.
Lewis, F.W., Harwood, L.M., and Hudson, M.J., WO Patent no. 2011 077 081, 2011.
Kozhevnikov, V.N., Kozhevnikov, D.N., Nikitina, T.V., Rusinov, V.L., Chupakhin, O.N., Zabel, M., and Koenig, B., J. Org. Chem., 2003, vol. 68, p. 2882.
Prokhorov, A.M., Kozhevnikov, V.N., Kopchuk, D.S., Bernard, H., Le Bris, N., Tripier, R., Handel, H., Koenig, B., and Kozhevnikov, D.N., Tetrahedron, 2011, vol. 67, p. 597.
Kozhevnikov, V.N., Kozhevnikov, D.N., Shabunina, O.V., Rusinov, V.L., and Chupakhin, O.N., Tetrahedron Lett., 2005, vol. 46, p. 1791.
Kopchuk, D.S., Krinochkin, A.P., Kozhevnikov, D.N., and Slepukhin, P.A., Polyhedron, 2016, vol. 118, p. 30.
Iqbal, N., Akula, M.R., Vo, D., Matowe, W.C., Mc-Ewen, C.-A., Wolowyk, M.W., and Knaus, E.E., J. Med. Chem., 1998, vol. 41, p. 1827.
Chigir’, A.N., Novikova, V.F., Kalikhman, I.D., Chumakov, Yu.I., Cherkashin, M.I., and Berlin, A.A., Polymer Sci. USSR, 1969, vol. 11, p. 2053.
Yamazaki, T., Saitou, A., Ono, M., Yokoyama, S., Bannai, K., Hirose, K., and Yanaka, M., EP Patent no. 1 431 290 A1, 2004.
Thallaj, N.K., Przybilla, J., Welter, R., and Mandon, D., J. Am. Chem. Soc., 2008, vol. 130, p. 2414.
Kozhevnikov, D.N., Kozhevnikov, V.N., Rusinov, V.L., Chupakhin, O.N., Sidorov, E.O., and Klyuev, N.A., Russ. J. Org. Chem., 1998, vol. 34, p. 393.
Chupakhin, O.N., Rusinov, V.L., Ulomsky, E.N., Kojevnikov, D.N., and Neunhoeffer, H., Mendeleev Commun., 1997, vol. 7, p. 66.
Dey, B.B., J. Chem. Soc., 1914, vol. 105, p. 1039.
Ashimori, A., Ono, T., Uchida, T., Ohtaki, Y., Fukaya, C., Watanabe, M., and Yokoyama, K., Chem. Pharm. Bull., 1990, vol. 38, p. 2446.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © A.P. Krinochkin, D.S. Kopchuk, N.V. Chepchugov, I.S. Kovalev, G.V. Zyryanov, V.L. Rusinov, O.N. Chupakhin, 2017, published in Zhurnal Organicheskoi Khimii, 2017, Vol. 53, No. 7, pp. 951–958.
Rights and permissions
About this article
Cite this article
Krinochkin, A.P., Kopchuk, D.S., Chepchugov, N.V. et al. Effect of substituent in pyridine-2-carbaldehydes on their heterocyclization to 1,2,4-triazines and 1,2,4-triazine 4-oxides. Russ J Org Chem 53, 963–970 (2017). https://doi.org/10.1134/S1070428017070016
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1070428017070016