Abstract
The capacity to oxidize sulfide and the influence of the simultaneous presence of acetate in heterotrophically (acetate) and autotrophically (sulfide/CO2) grown Rhodopseudomonas capsulata was investigated.
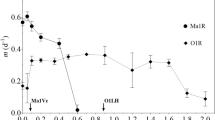
Sulfide oxidation of acetate-limited cultures was found inversely related to the specific growth rate. Upon acetate deprevation (metering pump stopped) increased rates of sulfide oxidation were observed. This points to the existence of a constitutive acceptor for the electrons from sulfide. It is suggested that a carrier functional in the light-induced cyclic electron flow operates as such. The rate of sulfide oxidation, however, is low when compared to autotrophically-grown cells. This is probably due to the low levels of Calvin cycle enzymes present in the acetate-grown cells.
In cells growing on sulfide/CO2, the addition of acetate resulted in less sulfide being oxidized. Upon depletion of the acetate, the rate of sulfide oxidation again increased, however, insufficiently to maintain the accelerated growth rate. This indicates that under mixotrophic conditions the enzymes of the Calvin cycle are being synthesized to a far lesser extent.
Similar content being viewed by others
Abbreviations
- PHB:
-
poly-β-hydroxybutyric acid
- D:
-
dilution rate
- TCA:
-
Tri carboxylic acid cycle
- RubPcase:
-
ribulose 1,5-bisphosphate carboxylase
- RP:
-
reducing power
References
Anderson L, Fuller RC (1967) Photosynthesis in Rhodospirillum rubrum. II. Photoheterophic carbon dioxide fixation. Plant Physiol 42:491–496
Beeftink HH, van Gemerden H (1979) Actual and potential rates of substrate oxidation and product formation in continuous culture of Chromatium vinosum. Arch Microbiol 121:161–167
Cappenberg ThE, Jongejan E (1978) Microenvironments for sulfate reduction and methane production in freshwater sediments. In: Krumbein WE (ed) Environm. Biogeochem. Geomicrobiol. Ann Arbor Sci Publ. Ann Arbor, 1 pp 129–138
Fischer U, Trüper HG (1977) Cytochrome c-550 of Thiocapsa roseopersicina: properties and reduction by sulfide. FEMS Lett 1:87–90
Hansen TA, van Gemerden H (1972) Sulfide utilization by purple nonsulfur bacteria. Arch Microbiol 86:49–56
Hansen TA (1974) Sulfide als electron donor voor Rhodospirillaceae. Ph. D. Thesis, University Groningen
Harder W, van Dijken JP (1976) Theoretical considerations on the relation between energy production and growth of methane utilizing bacteria. In: Schlegel HG, Pfennig N, Gottschalk G (eds) Proceedings Symposium on Microbial Production and Utilization of Gases. Goltze, Göttingen, pp 403–418
Herbert D, Phipps PJ, Strange RE (1971) Chemical analysis of microbial cells. In: Norris JR, Ribbons DW (eds) Methods in Microbiology. Vol 5B. Academic Press, New York, pp 209–344
Hurlbert RE, Lascelles J (1963) Ribulose diphosphate carboxylase in Thiorhodaceae. J Gen Microbiol 33:445–458
Kusai A, Yamanaka T (1973) Cytochrome c (553, Chlorobium thiosulfatophilum) is a sulphide-cytochrome c reductate. FEBS Lett 34:235–237
Law JH, Slepecky RA (1961) Assay of poly-hydroxybutyric acid. J Bacteriol 82:33–36
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Slater JH, Morris I (1973) Photosynthetic carbon dioxide assimilation by Rhodospirillum rubrum. Arch Mikrobiol 88:213–223
Stouthamer AH (1977) Energetic aspects of the growth of microorganisms. Symp Soc Gen Microbiol 27:285–315
Thauer RK, Jungermann K, Decker K (1977) Energy conservation in chemotrophic anaerobic bacteria. Bacteriol Rev 41:100–180
Trüper HG, Schlegel HG (1964) Sulphur metabolism in Thiorhodaceae. I. Quantitative measurements on growing cells of Chromatium okenii Antonie van Leeuwenhoek. J Microbiol Serol 30:225–238
Van Gemerden H, Beeftink HH (1978) Specific rates of substrate oxidation and product formation in autotrophically growing Chromatium vinosum cultures. Arch Microbiol 119:135–143
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Wijbenga, DJ., van Gemerden, H. The influence of acetate on the oxidation of sulfide by Rhodopseudomonas capsulata . Arch. Microbiol. 129, 115–118 (1981). https://doi.org/10.1007/BF00455344
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00455344