Abstract
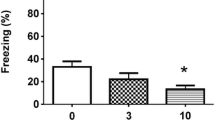
Neuronal plasticity can manifest itself in alterations in the sensitivity of memory to the effects of drugs. After the production of a brain lesion, the memory processing of a passive-avoidance task in mice gradually becomes sensitive to the effect of morphine, i.e., an improvement in retention performance is seen after 6 weeks, but not after 1 or 2 weeks. The results presented demonstrate that, even if they lead to no discernible changes in behaviour, plastic processes can still be detected by means of behavioural tests.
Similar content being viewed by others
References
Atweh SF, Kuhar MJ (1977) Autoradiographic localization of opiate receptors in rat brain. III. the telencephalon. Brain Res 134:393–405
Belluzzi JD, Stein L (1982) Brain endorphins: possible role in longterm memory. Ann NY Acad Sci 398:221–229
Castellano C, Pavone F (1985) Dose- and strain-dependent effects of demorphin and (d-Ala2-d-Leu5) enkephalin on passive avoidance behavior in mice. Behav Neurosci 99:1120–1127
Castellano C, Llovera BE, Oliverio A (1975) Morphine-induced running and analgesia in two strains of mice following septal lesions are modification of brain amines. Arch Pharmacol 288:355–370
Classen W, Mondadori C (1984) Facilitation or inhibition of memory by morphine: a question of experimental parameters. Experientia 40:506–508
Cotman CW, Nieto-Sampedro M (1984) Cell biology of synaptic plasticity. Science 225:1287–1294
Cotman CW, Matthews DA, Taylor D, Lynch GS (1973) Synaptic rearrangement in the dentate gyrus: histochemical evidence of adjustments after lesions in immature and adult rats. Proc Natl Acad Sci USA 70:3473–3477
Crutcher KA, Kesner RP, Novak JM (1983) Medial septal lesions, radial arm maze performance, and sympathetic sprouting: a study of recovery of function. Brain Res 262:91–98
Donovick PJ, Burright RG, Bengelloun WA (1979) The septal region and behavior: an example of the importance of genetic and experiential factors in determining effects of brain damage. Neurosci Biobehav Rev 3:83–96
Epstein AN (1971) The lateral hypothalamus syndrome: its implication for the physiological psychology of hunger and thirst. Prog Physiol Psychol 4:263–318
Fowler KR, Olton DS (1984) Recovery of function following injections of kainic acid: behavioral, electrophysiological and neuroanatomical correlates. Brain Res 321:21–32
Gage FH, Björklund A, Stenevi U (1983) Reinnervation of the partially deafferented hippocampus by compensatory collateral sprouting from spared cholinergic and noradrenergic afferents. Brain Res 268:27–37
Geddes JW, Monaghan DT, Cotman CW, Lott IT, Kim RC, Chui HC (1985) Plasticity of hippocampal circuitry in Alzheimer's disease. Science 230:1179–1181
Harrell LE, Barlow TS, Davis JN (1983) Sympathetic sprouting and recovery of a spatial behavior. Exp Neurol 82:379–390
Irle E (1987) Lesion size and recovery of function: some new perspectives. Brain Res Rev 12:307–320
Jensen RA, Martinez JL, Messing RB, Spiehler V, Vasquez BJ, Soumireu-Mourat B, Liang KD, McGaugh JL (1978) Morphine and naloxone alter memory in the rat. Soc Neurosci Abstr 4:260
Kuhar MJ, Pert CB, Snyder SH (1973) Regional distribution of opiate receptor binding in monkey and human brain. Nature 245:447–450
Lynch G, Matthews DA, Mosko S, Parks T, Cotman C (1972) Induced acetylcholinesterase-rich layer in rat dentate gyrus following entorhinal lesions. Brain Res 42:311
Martinez JL Jr, Jensen RA, Messing RB, Rigter H, McGaugh JL (1981) Endogenous peptides and learning and memory processes. Academic Press, New York, p 587
Matthews DA, Cotman CW, Lynch GS (1976) An electron microscopic study of lesion-induced synaptogenesis in the dentate gyrus of the adult rat. I. Magnitude and time course of degeneration. Brain Res 115:1–21
Matthews DA, Cotman CW, Lynch GS (1976) An electron microscopic study of lesion-induced synaptogenesis in the dentate gyrus of the adult rat. II. Reappearance of morphologically normal contacts. Brain Res 115:23–41
McDaniel JR, Donovick PJ, Burright RG, Fanelli RJ (1980) Genetics, septal lesions and avoidance behavior in mice. Behav Neural Biol 28:285–299
McGaugh JL, Herz M (1972) Memory consolidation. Albion, San Francisco
Mondadori C (1981) Pharmacological modulation of memory: trends and problems. Acta Neurol Scand [Suppl 64] 89:129–136
Mondadori C (1987) Pharmacology of memory — science or art? In: Mutschler E, Winterfeldt E (eds) Trends in medicinal chemistry. Proceedings of the 9th Int Symposium on Medicinal Chemistry, Berlin 1986
Powers B, Valenstein ES (1972) Sexual receptivity: facilitation by medial preoptic lesions in female rats. Science 175:1003–1005
Slotnick BM, Jarvik ME (1966) Deficits in passive avoidance and fear conditioning in mice with septal lesions. Science 154:1207–1208
Slotnick BM, Leonard CMA (1975) Stereotaxic atlas of the albino mouse forebrain. US Dept of Health Education and Welfare, Rockville
Slotnick BM, Nigrosh BJ (1975) Maternal behavior of mice with cingulate cortical, amygdala, or septal lesions. J Comp Physiol Psychol 88:118–127
Storm-Mathisen J (1974) Choline acetyltransferase and acetylcholinesterase in fascia dentata following lesion of the entorhinal afferents. Brain Res 80:181–197
Tukey JW (1977) Exploratory data analysis. Addison-Wesley, London, pp 47
White N, Major R, Siegel J (1978) Effects of morphine on one-trial appetitive learning. Life Sci 23:1967–1972
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Mondadori, C., Back, M. Neural plasticity in vivo: opioid sensitivity of memory develops gradually after a septal lesion. Psychopharmacology 99, 294–298 (1989). https://doi.org/10.1007/BF00445546
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00445546