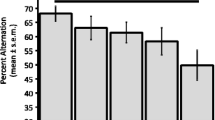
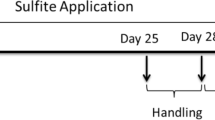
Abstract
Common marmosets (Callithrix jacchus) were trained to perform daily position discrimination learning tasks in a Wisconsin General Test Apparatus. Acetylcholine receptor blockade with scopolamine was found to impair position learning. Testing on the day after scopolamine treatment suggested that a task learnt under scopolamine was not encoded into long term memory. Acetylcholine depletion achieved by the intraventricular injection of hemicholinium 4 h before testing resulted in a profound impairment of position discrimination learning. It is suggested that central acetylcholine depletion in primates may provide a useful model of senile dementia.
Similar content being viewed by others
References
Bartus RT, Johnson HR (1976) Short term memory in the rhesus monkey: disruption from the anticholinergic scopolamine. Pharmacol Biochem Behav 5:39–46
Birks RI, MacIntosh FC (1961) Acetylcholine metabolism of a sympathetic ganglion. Can J Biochem Physiol 39:787–827
Bohdanecky Z, Jarvik ME, Carley JL (1967) Differential impairment of delayed matching in monkeys by scopolamine and scopolamine methylbromide. Psychopharmacologia 11:293–299
Caulfield MP, May PJ, Pedder EK, Prince AK (1983) Behavioural studies with ethylcholine mustard aziridinium (ECMA). Br J Pharmacol 79:287P
Cotterman TE, Meyer DR, Wickens DD (1956) Discrimination reversal learning in marmosets. J Comp Physiol Psychol 49:539–541
Crow TJ, Grove-White IG (1973) An analysis of the learning deficit following hyoscine administration to man. Br J Pharmacol 49:322–327
Davies P, Maloney AJ (1976) Selective loss of central cholinergic neurones in Alzheimer's disease. Lancet II:1430
Davies P, Verth AH (1978) Regional distribution of muscarinic acethylcholine receptors in normal and Alzheimer's type dementia brains. Brain Res 138:385–392
Davis RT (1956) Problem-solving behavior of monkeys as a function of work variables. J Comp Physiol Psychol 49:499–506
Drachman DA (1977) Memory and cognitive function in man: does the cholinergic system have a specific role? Neurology 27:783–790
Evans HL (1975) Scopolamine effects on visual discrimination: modifications related to stimulus control. J Pharmacol Exp Ther 195:105–109
Freeman JJ, Macri JR, Choi RL, Jenden DJ (1979) Studies on the behavioral and biochemical effects of hemicholinium in vivo. J Pharmacol Exp Ther 210:91–97
Gellerman LW (1933) Chance orders of alternating stimuli in visual discrimination experiments. J Genet Psychology 42:206–208
Glick SD, Jarvik ME (1969) Amphetamine, scopolamine and chlorpromazine interactions on delayed matching performance in monkeys. Psychopharmacologia 16:147–155
Harlow HF, Bromer JA (1938) A test-apparatus for monkeys. Psychol Rec 2:434–436
Jacobsen CF, Wolfe JB, Jackson TA (1935) An experimental analysis of the functions of the frontal association area in primates. J Nerv Ment Dis 83:1–4
Malmo RB (1942) Interference factors in delayed response in monkeys after removal of frontal lobes. J Neurophysiol 5:295–308
Mewaldt SP, Ghoneim MM (1979) The effects and interactions of scopolamine, physostigmine and methamphetamine on human memory. Pharmacol Biochem Behav 10:205–210
Meyer DR (1951) Food deprivation and discrimination reversal learning in the monkey. J Exp Psychol 44:10–16
Meyer DR, Treichler FR, Meyer PM (1965) Discrete-trial training techniques and stimulus variables. In: Schrier AM, Harlow HF, Stollnitz F (eds) Behaviour of non-human primates, vol. 1. Academic Press, New York, pp 1–49
Miles RC (1959) Discrimination in the squirrel monkey as a function of deprivation and problem difficulty. J Exp Psychol 57:15–19
Miles RC, Meyer DR (1956) Learning sets in marmosets. J Comp Physiol Psychol 49:219–222
Mishkin M (1964) Perseveration of central sets after frontal lesions in monkeys. In: Warren JM, Akert K (eds) The frontal granular cortex and behaviour. McGraw-Hill, New York, pp 219–241
Mishkin M, Spiegler BJ, Saunders RC, Malamut BL (1982) An animal model of global amnesia. In: Corkin S, Davis KL, Growde JH, Usdin E, Wurtman RJ (eds) Alzheimer's disease: a review of progress. Raven Press, New York, pp 235–247
Overton DA (1964) State-dependent or “dissociated” learning produced with pentobarbital. J Comp Physiol Psychol 57:3–12
Perry EK, Perry RH, Blessed G, Tomlinson BE (1977) Necropsy evidence of central cholinergic deficits in senile dementia. Lancet I:189
Perry EK, Tomlinson BE, Blessed G, Bergmann K, Gibson PH, Perry RH (1978) Correlation of cholinergic abnormalities with senile plaques and mental test scores in senile dementia. Br Med J 2:1457–1459
Ridley RM, Bowes PM, Baker HF, Crow TJ (1984) An involvement of acetylcholine in object discrimination learning and memory in the marmoset. Neuropsychologia in press
Russell RW, Macri J (1978) Some behavioral effects of suppressing choline transport by cerebroventricular injection of hemicholinium-3. Pharmacol Biochem Behav 8:399–403
Safer DJ, Allen RP (1971) The central effects of scopolamine in man. Biol Psychiatry 3:347–355
Stephan H, Baron G, Schwerdtfeyer WK (1980) The brain of the common marmoset: a stereotaxic atlas. Springer, Berlin Heidelberg New York
Wilcock GK, Esiri MM, Bowen DM, Smith CCT (1982) Alzheimer's disease: correlation of cortial choline acetyltransferase activity with the severity of dementia and histological abnormalities. J Neurol Sci 57:407–417
Yamamura HI, Snyder SH (1973) High affinity transport of choline into synaptosomes of rat brain. J Neurochem 21:1355–1374
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ridley, R.M., Barratt, N.G. & Baker, H.F. Cholinergic learning deficits in the marmoset produced by scopolamine and ICV hemicholinium. Psychopharmacology 83, 340–345 (1984). https://doi.org/10.1007/BF00428542
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00428542