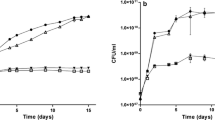
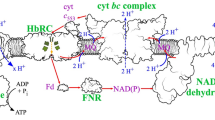
Abstract
We present an extended genetic analysis of the previously identified cycH locus in Bradyrhizobium japonicum. Three new open reading frames found in an operon-like structure immediately adjacent to the 3′ end of cycH were termed cycJ, cycK and cycL. A deletion mutant (ΔcycHJKL) and biochemical analysis of its phenotype showed that the genes of the cluster are essential for the biogenesis of cellular c-type cytochromes. Mutations in discrete regions of each of the genes were also constructed and shown to affect anaerobic respiration with nitrate and the ability to elicit an effective symbiosis with soybean, both phenotypes being a consequence of defects in cytochrome c formation. The CycK and CycL proteins share up to 53% identity in amino acid sequence with the Rhodobacter capsulatus Ccll and Cc12 proteins, respectively, which have been shown previously to be essential for cytochrome c biogenesis, where-as cycJ codes for a novel protein of 169 amino acids with an Mr of 17857. Localisation studies revealed that CycJ is located in the periplasmic space; it is probably anchored to the cytoplasmic membrane via an N-terminal hydrophobic domain. Based on several considerations discussed here, we suggest that the proteins encoded by the cycHJKL-cluster may be part of a cytochrome c-haem lyase complex whose active site faces the periplasm.
Similar content being viewed by others
References
Amersham International plc. (1984) M13 cloning and sequencing handbook. Amersham, Buckinghamshire, UK
Appleby CA (1984) Leghemoglobin and Rhizobium respiration. Annu Rev Plant Physiol 35:443–478
Bardwell JCA, McGovern K, Beckwith J (1991) Identification of a protein required for disulfide bond formation in vivo. Cell 67:581–589
Beckman DL, Kranz G (1993) Cytochrome c biogenesis in a photosynthetic bacterium requires a periplasmic thioredoxin-like protein. Proc Natl Acad Sci USA 90:2179–2183
Beckman DL, Trawick DR, Kranz G (1992) Bacterial cytochrome c biogenesis. Genes Dev 6:268–283
Blattner FR, Burland V, Plunkett G, Sofia HJ, Daniels DL (1993) Analysis of the Escherichia coli genome. IV. DNA sequence of the region from 89.2 to 92.8 minutes. Nucleic Acids Res 21:5408–5417
Bott M, Bolliger M, Hennecke H (1990) Genetic analysis of the cytochrome c-aa 3 branch of the Bradyrhizobium japonicum respiratory chain. Mol Microbiol 4:2147–2157
Bott M, Ritz D, Hennecke H (1991) The Bradyrhizobium japonicum cycM gene encodes a membrane-anchored homolog of mitochondrial cytochrome c. J Bacteriol 173:6766–6772
Brickman E, Beckwith J (1975) Analysis of the regulation of Escherichia coli alkaline phosphatase synthesis using deletions and ϕ80 transducing phages. J Mol Biol 96:307–316
Creusot F, Verdière J, Gaisne M, Slonimski PP (1988) CYP1 (HAP1) regulator of oxygen-dependent gene expression in yeast. J Mol Biol 204:263–276
Daniel RM, Appleby CA (1972) Anaerobic-nitrate, symbiotic and aerobic growth of Rhizobium japonicum: effects on cytochrome P450, other haemoproteins, nitrate and nitrite reductases. Biochim Biophys Acta 275:347–354
Darwin A, Tormay P, Page L, Griffiths L, Cole J (1993a) Identification of the formate dehydrogenases and genetic determinants of formate-dependent nitrite reduction by E. coli K12. J Gen Microbiol 139:1829–1840
Darwin A, Hussain H, Griffiths L, Grove J, Sambongi Y, Busby S, Cole J (1993b) Regulation and sequence of the structural gene for the cytochrome C 552 from Escherichia coli: not a hexahaem but a 50 kDa tetrahaem nitrite reductase. Mol Microbiol 9:1255–1265
Drygas ME, Lambowitz AM, Nargang FE (1989) Cloning and analysis of the Neurospora crassa gene for cytochrome-c-heme lyase. J Biol Chem 264:17897–17906
Dumont ME, Ernst JF, Hampsey MD, Sherman F (1987) Identification and sequence of the gene encoding cytochrome-c-heme lyase in the yeast Saccharomyces cerevisiae. EMBO J 6:235–241
Eisenberg D, Schwarz E, Komarony M, Wall R (1984) Analysis of membrane and surface protein sequences with the hydrophobic moment plot. J Mol Biol 179:125–142
Ferguson SJ (1988) Periplasmic electron transport reactions. In: Anthony C (ed) Bacterial energy transduction. Academic Press, London, pp 151–182
Francis RT, Becker RR (1984) Specific indication of hemoproteins in polyacrylamide gels using a double-staining process. Anal Biochem 136:509–514
Fuhrmann M, Hennecke H (1982) Coding properties of cloned nitrogenase structural genes from Rhizobium japonicum. Mol Gen Genet 187:417–425
Gleason FK, Holmgren A (1988) Thioredoxin and related proteins in prokaryotes. FEMS Microbiol Rev 54:271–298
Gonzales DH, Neupert W (1990) Biogenesis of mitochondrial ctype cytochromes. J Bioenerg Biomembr 22:753–768
Gonzales DH, Bonnard G, Grienenberger J-M (1993) A gene involved in the biogenesis of c-type cytochromes is co-transcribed with a ribosomal protein gene in wheat mitochondria. Curr Genet 24:248–255
Göttfert M, Holzhdäser D, Böni D, Hennecke H (1992) Structural and functional analysis of two different nodD genes in Bradyrhizobium japonicum USDAl10. Mol Plant-Microbe Interact 5:257–265
Hahn M, Hennecke H (1984) Localized mutagenesis in Rhizobium japonicum. Mol Gen Genet 193:46–52
Hanahan D (1983) Studies on transformation of Escherichia coli with plasmids. J Mol Biol 166:557–563
Hennecke H, Anthamatten D, Babst M, Bott M, Fischer HM, Kaspar T, Kullik I, Loferer H, Preisig O, Ritz D, Weidenhaupt M (1993) Genetic and physiologic requirements for optimal bacteroid function in the Bradyrhizobium japonicum- soybean symbiosis. In: Nester EW, Verma DPS (eds) Advances in molecular genetics of plant-microbe interactions. Kluwer, Dordrecht, pp 199–207
Hoffman CS, Wright A (1985) Fusions of secreted proteins to alkaline phosphatase: an approach for studying protein secretion. Proc Natl Acad Sci USA 82:5107–5111
Hussain H, Grove J, Griffiths L, Busby S, Cole J (1994) A sevengene operon essential for formate-dependent nitrite reduction to ammonia by enteric bacteria. Mol Microbiol 12:153–163
Kereszt A, Slaska-Kiss K, Putnoky P, Banfalvi Z, Kondorosi A (1995) Rhizobium meliloti cycHJKL genes involved in cytochrome c biogenesis are required for respiratory nitrate reduction ex planta and for nitrogen fixation in symbiosis. Mol Gen Genet 247:39–47
Kitto GB (1969) Intra- and extramitochondrial malate dehydrogenate from chicken and tuna heart. Methods Enzymol 13:106–107
Klein P, Kanehisa M, DeLisi C (1985) The detection and classification of membrane-spanning proteins. Biochim Biophys Acta 815:468–476
Kündig C, Hennecke H, Göttfert M (1993) Correlated physical and genetic map of the Bradyrhizobium japonicum strain 110 genome. J Bacteriol 175:613–622
Layzell DB, Hunt S, Moloney AHM, Fernando SM, Diaz del Castillo, L (1990) Physiological, metabolic and developmental implications of O2 regulation in legume nodules. In: Gresshoff PM, Roth LE, Stacey G, Newton WE (eds) Nitrogen fixation: achievements and objectives. Chapman and Hall, New York and London, pp 21–32
Lill R, Stuart RA, Drygas ME, Nargang FE, Neupert W (1992) Import of cytochrome c heme lyase into mitochondria: a novel pathway into the intermembrane space. EMBO J 11:449–456
Loferer H, Bott M, Hennecke H (1993) Bradyrhizobium japonicum TlpA, a novel membrane-anchored thioredoxin-like protein involved in the biogenesis of cytochrome aa 3 and development of symbiosis. EMBO J 12:3373–3383
Manoil C, Mekalanos JJ, Beckwith J (1990) Alkaline phosphatase fusions: sensors of subcellular location. J Bacteriol 172:515–518
Messing J (1983) New M13 vectors for cloning. Methods Enzymol 101:20–78
Nicholson DW, Köhler H, Neupert W (1987) Import of cytochrome c into mitochondria. Eur J Biochem 164:147–157
Nicholson DW, Stuart RA, Neupert W (1989) Biogenesis of cytochrome c 1. J Biol Chem 264:10156–10168
Norrander J, Kempe T, Messing J (1983) Construction of improved M13 vectors using oligonucleotide-directed mutagenesis. Gene 26:101–106
Oda K, Yamato K, Ohta E, Nakamura Y, Takemura M, Nozato N, Akashi K, Kanegae T, Ogura Y, Kohchi T, Ohyama K (1992) Gene organization deduced from the complete sequence of liverwort Marchantia polymorpha mitochondrial DNA. J Mol Biol 223:1–7
Ohayama K, Fukuzawa H, Kohchi T, Shirai H, Sano T, Sano S, Umesono K, Shiki Y, Tkeuchi M, Chang Z, Aota S, Inokuchi H, Ozeki H (1986) Chloroplast gene organization deduced from complete sequence of liverwort Marchantia polymorpha chloroplast DNA. Nature 322:572–574
Page MD, Ferguson SJ (1989) A bacterial c-type cytochrome can be translocated to the periplasm as an apo form; the biosynthesis of cytochrome cd 1 (nitrite reductase) from Paracoccus denitrificans. Mol Microbiol 3:653–661
Page MD, Ferguson SJ (1990) Apo forms of cytochrome C 550 and cytochrome cd 1 are translocated to the periplasm of Paracoccus denitrificans in the absence of haem incorporation caused by either mutation or inhibition of haem synthesis. Mol Microbiol 4:1181–1192
Pettigrew GW, Moore GR (1987) Cytochromes c. Springer, Berlin, pp 160–179
Pfeifer K, Kim K-S, Kogan S, Guarente L (1989) Functional dissection and sequence of yeast HAP1 activator. Cell 56:291–301
Preisig O, Anthamatten D, Hennecke H (1993) Genes for a novel, microaerobically induced oxidase complex in Bradyrhizobium japonicum are essential for a nitrogen-fixing endosymbiosis. Proc Natl Acad Sci USA 90:3309–3313
Ramseier TM, Göttfert M (1991) Codon usage and G+C content in Bradyrhizobium japonicum genes are not uniform. Arch Microbiol 156:270–276
Ramseier TM, Winteler HV, Hennecke H (1991) Discovery and sequence analysis of bacterial genes involved in the biogenesis of c-type cytochromes. J Biol Chem 266:7793–7803
Rao MJK, Argos P (1986) A conformational preference parameter to predict helices in integral membrane proteins. Biochim Biophys Acta 869:197–214
Regensburger B, Hennecke H (1983) RNA polymerase from Rhizobium japonicum. Arch Microbiol 135:103–109
Ritz D, Bott M, Hennecke H (1993) Formation of several bacterial c-type cytochromes requires a novel membrane-anchored protein that faces the periplasm. Mol Microbiol 9:729–740
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning — a laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, New York
Sanger F, Nicklen S, Coulson AR (1977) DNA sequencing with chain terminating inhibitors. Proc Natl Acad Sci USA 74:5463–5467
Schuster W, Combettes B, Flieger K, Brennicke A (1993) A plant mitochondrial gene encodes a protein involved in cytochrome c biogenesis. Mol Gen Genet 239:49–57
Simon R, Priefer U, Pühler A (1983) Vector plasmids for vivo and vitro manipulation of Gram-negative bacteria. In: Pühler A (ed) Molecular genetics of the bacteria-plant interaction. Springer, Berlin, pp 98–106
Taniuchi H, Basile G, Taniuchi M, Veloso D (1983) Evidence for formation of two thioether bonds to link heme to apocytochrome c by partially purified cytochrome c synthetase. J Biol Chem 258:10963–10966
Thöny B, Kaluza K, Hennecke H (1985) Structural and functional homology between the α and β subunits of the nitrogenase MoFe protein as revealed by sequencing the Rhizobium japonicum nifK gene. Mol Gen Genet 198:441–448
Thöny-Meyer L, Stax D, Hennecke H (1989) An unusual gene cluster for the cytochrome bc 1 complex in Bradyrhizobium japonicum and its requirement for effective root nodule symbiosis. Cell 57:683–697
Thöny-Meyer L, James P, Hennecke H (1991) From one gene to two proteins: the biogenesis of cytochromes b and c 1 in Bradyrhizobium japonicum. Proc Natl Acad Sci USA 88:5001–5005
Thöny-Meyer L, Ritz D, Hennecke H (1994) Cytochrome c biogenesis in bacteria: a possible pathway begins to emerge. Mol Microbiol 12:1–9
Tinoco I, Borer PN, Dengler B, Levine MD, Uhlenbeck OC, Crothers DM, Gralla J (1973) Improved estimation of secondary structure in ribonucleic acids. Nature 246:40–41
Turner GL, Gibson AH (1980) Measurement of nitrogen fixation by indirect means. In. Bergersen FJ (ed) Methods for evaluating biological nitrogen fixation. John Wiley and Sons, Chichester, pp 111–138
von Heijne G (1986) The distribution of positively charged residues in bacterial inner membrane proteins correlates with the trans-membrane topology. EMBO J 5:3021–3027
von Heijne G (1988) Transcending the impenetrable: how proteins come to terms with membranes. Biochim Biophys Acta 947:307–333
von Wachenfeldt C, Hederstedt L (1990) Bacillus subtilis 13-kilodalton cytochrome c-550 encoded by cccA consists of a membrane-anchor and a heme domain. J Biol Chem 265:147–151
Wood PM (1983) Why do c-type cytochromes exist? FEBS Lett 164:223–226
Wood PM (1991) Why do c-type cytochromes exist? - Reprise. Biochim Biophys Acta 1058:5–7
Zollner A, Rödel G, Haid A (1992) Molecular cloning and characterization of the Saccharomyces cerevisiae CYT2 gene encoding cytochrome-c 1-heme lyase. Eur J Biochem 207:1093–1100
Author information
Authors and Affiliations
Additional information
Communicated by J. Lengeler
Rights and permissions
About this article
Cite this article
Ritz, D., Thöny-Meyer, L. & Hennecke, H. The cycHJKL gene cluster plays an essential role in the biogenesis of c-type cytochromes in Bradyrhizobium japonicum . Molec. Gen. Genet. 247, 27–38 (1995). https://doi.org/10.1007/BF00425818
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF00425818