Abstract
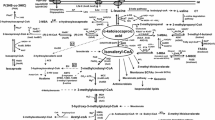
α-Isopropylmalate synthase (EC 4.1.3.12) is present in extracts of Bacteroides fragilis, Clostridium thermoaceticum, Clostridium formicoacetium, Clostridium pasteurianum, and Clostridium kluyveri with specific activities (μmol α-isopropylmalate formed per min and g protein) of 8.6, 8.9, 2.4, 1.9, and 0.3, respectively. The product α-isopropylmalate was identified by gas chromatography combined with mass spectroscopy. The presence of 5 mM leucine in the growth medium represses the synthesis of α-isopropylmalate synthase in C. thermoaceticum by 40 and 70 %. The enzyme from C. pasteurianum was partially purified to a specific activity of 1413. All studied enzyme properties are similar to those of the enzymes from aerobic bacteria. It is suggested that in these anaerobic bacteria the α-isopropylmalate pathway is present in addition to the pathway via the ferrodoxin-dependent, reductive carboxylation of branched chain fatty acids.
Similar content being viewed by others
Abbreviations
- α-KIV:
-
α-Ketoisovalerate
- α-IPM:
-
α-Isopropylmalate
- CoA:
-
Coenzyme A
References
Abelson PH, Vogel HJ (1955) Amino acid biosynthesis in Torulopsis utilis and Neurospora crassa. J Biol Chem 213:335–364
Allison MJ (1969) Biosynthesis of amino acids by ruminal microorganisms. J Anim Sci 29:797–807
Allison MJ, Bryant MP (1963) Biosynthesis of branched-chain amino acids from branched-chain fatty acids by rumen bacteria. Arch Biochem 101:269–277
Allison MJ, Bucklin JA, Robinson IM (1966) Importance of the isovalerate carboxylation pathway of leucine biosynthesis in the rumen. Appl Microbiol 14:807–814
Beisenherz G, Bolze HJ, Bücher Th, Czok R, Garbade HK, Mayer-Arendt E, Pfleiderer G (1953) Diphosphofructose-Aldolase, Phosphoglyceraldehyd-Dehydrogenase, Milchsäure-Dehydrogenase und Pyruvat-Kinase aus Kaninchenmuskulatur in einem Arbeitsgang. Z Naturf 8 B:555–577
Berndt H, Schlegel HG (1975) Kinetics and properties of α-ketothiolase from Clostridium pasteurianum. Arch Microbiol 103:21–30
Buchanan BB (1972) Ferredoxin-linked carboxylation reactions. In: Boyer PD (ed) The enzymes, vol VI, (3rd ed), Academic Press, New York and London, pp 193–216
Calvo JM, Gross SR (1970) Isolation and chemical estimation of α-isopropylmalate and β-isopropylmalate. In: Tabor H, Tabor CW (eds) Methods in enzymology, vol 17A. Academic Press Inc., New York, pp 791–793
Himes RH, Harmony JAK (1973) Formyltetrahydrofolate synthetase. Crit Rev Biochem 1:501–535
Kohlhaw G, Leary TR, Umbarger HE (1969) α-Isopropylmalate synthase from Salmonella typhimurium. Purification and properties. J Biol Chem 244:2218–2225
Langenbeck U, Mohring HU, Dieckmann KP (1975) Gas chromatography of α-keto acids as their o-trimethylsilyl quinoxalinol derivatives. J Chromat 115:65–70
Ljungdahl LG, Sherod DW, Moore MR, Andreesen JR (1976) Properities of enzymes from Clostridium thermoaceticum and Clostridium formicoaceticum. In: Zuber H (ed) Enzymes and proteins from thermophilic microorganisms. Birkhäuser Verlag, Basel, pp 237–248
Macy J, Probst I, Gottschalk G (1975) Evidence for cytochrome involvement in fumarate reduction and adenosine 5′-triphosphate synthesis by Bacteroides fragilis grown in the presence of hemin. J Bacteriol 123:436–442
Miflin BJ, Cave PR (1972) The control of leucine, isoleucine and valine biosynthesis in a range of higher plants. J Exp Bot 23:511–516
Ruhr E (1977) Regulation der Biosynthese von Poly-\-hydroxybuttersäure in Alcaligenes eutrophys H 16. Ph. D. thesis Univ Göttingen
Sauter FD, Erfle JD, Mahadevan S (1975) Amino acid biosynthesis in mixed rumen cultures. Biochem J 150:357–372
Simon EJ, Shemin D (1953) The preparation of S-succinyl-coenzyme A. J Amer Chem Soc 75:2520
Singer RA, Doolittle WF (1975) Leucine biosynthesis in the bluegreen bacterium Anacystis nidulans. J Bacteriol 124:810–814
Stadtman ER, Burton RM (1955) Aldehyde dehydrogenase from Clostridium kluyweri. In: Colowick SP, Kaplan NO (ess) Methods in enzymology, vol 1. Academic Press, New York, pp 518–523
Stieglitz BI, Calvo JM (1974) Distribution of the isopropylmalate pathway to leucine among diverse bacteria. J Bacteriol 118:935–941
Strassman M, Locke LA, Thomas AJ, Weinhouse S (1956) A study of leucine biosynthesis in Torulopsis utilis. J Amer Chem Soc 78:1599–1602
Ulm EH, Bohme R, Kohlhaw G (1972) α-Isopropylmalate synthase from yeast: Purification, kinetic studies, and effect of ligands on stability. J Bacteriol 110:118–126
Umbarger HE (1978) Amino acid biosynthesis and its regulation. Ann Rev Biochem 47:533–606
Wiegel J (1973) Die α-Isopropylmalat-Synthase in Hydrogenomonas eutropha H 16. Ph. D. thesis Univ Göttingen
Wiegel J (1978) Mn2+-specific reactivation of EDTA inactivated α-isopropylmalate synthase from Alcaligenes eutrophus H16. Biochem Biophys Res Commun 82:907–912
Wiegel J, Schlegel HG (1977a) α-Isopropylmalate synthase from Alcaligenes eutrophus H16. I. Purification and general properties. Arch Microbiol 112:239–246
Wiegel J, Schlegel HG (1977b) α-Isopropylmalate synthase from Alcaligenes eutrophus H16. II. Substrate specificity and kinetics. Arch Microbiol 112:247–254
Wiegel J, Schlegel HG (1977c) α-Isopropylmalate synthase in Alcaligenes eutrophus H16. III. End product inhibition and its relief by valine and isoleucine. Arch Microbiol 114:203–210
Wiegel J, Schlegel HG (1977d) Leucine biosynthesis: Effect of leucine, valine, isoleucine and threonine on α-isopropylmalate synthase activity from aerobic and anaerobic microorganisms. Biochem Syst Ecol 5:169–176
Wixom RL, Wikman JH, Howell GB (1961) Valine biosynthesis. III. Biological distribution of a dihydroxy acid dehydrase. J Biol Chem 236:3257–3262
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Wiegel, J. α-Isopropylmalate synthase as a marker for the leucine biosynthetic pathway in several clostridia and in Bacteroides fragilis . Arch. Microbiol. 130, 385–390 (1981). https://doi.org/10.1007/BF00414605
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00414605