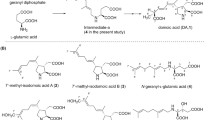
Summary
-
1.
The “biosynthetic” l-threonine (deaminating) dehydratase activity of 7 marine planktonic species from 5 classes of algae showed high substrate specificity toward l-threonine, with the exception of one alga. The algal extracts deaminated l-serine and l-allothreonine at rates which were 20–25 and 5–10%, respectively, of that of l-threonine, and these reaction were inhibited by l-isoleucine. d-Threonine, d-serine, l-homoserine, and l-O-methylthreonine were ineffective as substrates.
-
2.
Extracts of the cryptophyte Chroomonas salina were exceptional in showing about twice the activity with l-serine relative to l-threonine. The reaction with l-serine was insensitive to inhibition by l-isoleucine and differed, in several respects from that with l-threonine. It was inferred that this algal extract contains a specific l-serine deaminase in addition to the regular l-threonine deaminase.
-
3.
The rate of deamination of l-threonine by the algal extracts was not affected by the simultaneous presence of l-allothreonine but was markedly inhibited by l-serine and l-homoserine.
-
4.
Kinetic studies of the effects of graded concentrations of the substrate analogs at a fixed (saturating) substrate level or of substrate concentration at fixed analog levels indicated that l-serine inhibited the threonine deamination by irreversible competition with the substrate, whilst l-homoserine affected this reaction by an unknown (presumably allosteric) mechanism not involving substrate competition.
Similar content being viewed by others

Abbreviations
- TD:
-
l-Threonine dehydratase (deaminase)
- SD:
-
l-serine dehydratase (deaminase)
References
Antia, N. J., Cheng, J. Y.: The survival of axenic cultures of marine planktonic algae from prolonged exposure to darkness at 20°C. Phycologia 9, 179–184 (1970)
Antia, N. J., Kripps, R. S., Desai, I. D.: l-Threonine deaminase in marine planktonic algae. II. Disulfide and sulfhydryl group requirements of enzyme activity in two cryptophytes. J. Phycol. 8, 283–289 (1972a)
Antia, N. J., Kripps, R. S., Desai, I. D.: l-Threonine deaminase in marine planktonic algae. III. Stimulation of activity by monovalent inorganic cations and diverse effects from other ions. Arch. Mikrobiol. 85, 341–354 (1972b)
Carter, J. E., Sagers, R. D.: Ferrous ion-dependent l-serine dehydratase from Clostridium acidiurici. J. Bact. 109, 757–763 (1972)
Cennamo, C., Boll, M., Holzer, H.: Über Threonindehydratase aus Saccharomyces cerevisiae. Biochem. Z. 340, 125–145 (1964)
Changeux, J.-P.: Sur les propriétés allostériques de la l-thréonine désaminase de biosynthèse. III. Interprétation de l'effet inhibiteur de la l-isoleucine: empêchement stérique ou effet allostérique. Bull. Soc. Chim. biol. (Paris) 46,1151–1173 (1964)
Dart, R. K.: The presence of threonine and serine dehydratase activities in Pseudomonas. Biochem. J. 107, 29P-30P (1968)
Datta, P.: Regulation of branched biosynthetic pathways in bacteria. Science 165, 556–562 (1969a)
Datta, P.: Effects of feedback modifiers on mutationally altered threonine deaminases of Rhodopseudomonas spheroides. J. biol. Chem. 244, 858–864 (1969b)
Desai, I. D., Laub, D., Antia, N. J.: Comparative characterization of l-threonine dehydratase in seven species of unicellular marine algae. Phytochem. 11, 277–287 (1972)
Dougall, D. K.: Threonine deaminase from Paul's Scarlet Rose tissue cultures. Phytochem. 9, 959–964 (1970)
Dupourque, D., Newton, W. A., Snell, E. E.: Purification and properties of d-serine dehydrase from Escherichia coli. J. biol. Chem. 241, 1233–1238 (1966)
Feldberg, R. S., Datta, P.: l-Threonine deaminase of Rhodospirillum rubrum. Purification and characterization. Europ. J. Biochem. 21, 438–446 (1971)
Freundlich, M., Umbarger, H. E.: The effects of analogs of threonine and of isoleucine on the properties of threonine deaminase. Cold Spr. Harb. Symp. quant. Biol. 28, 505–511 (1963)
Friedemann, T. E.: Determination of α-keto acids, pp. 414–418. In:S. P. Colowick, N. O. Kaplan, Eds., Methods in enzymology, Vol.3. New York: Academic Press 1957
Guillard, R. R. L., Ryther, J. H.: Studies of marine planktonic diatoms. I. Cyclotella nana Husted, and Detonula confervaceae (Cleve) Gran. Canad. J. Microbiol. 8, 229–239 (1962)
Hasle, G. R., Heimdal, B. R.: Some species of the centric diatom genus Thalassiosira studied in the light and electron microscopies. Nova Hedwigia 31, 559–581 (1970)
Hatfield, G. W., Burns, R. O.: Ligand-induced maturation of threonine deaminase. Science 167, 75–76 (1970)
Hill, H. M., Rogers, L. J.: Bacterial origin of alkaline l-serine dehydratase in french beans. Phytochem. 11, 9–18 (1972)
Hirata, M., Tokushige, M., Inagaki, A., Hayaishi, O.: Nucleotide activation of threonine deaminase from Escherichia coli. J. biol. Chem. 240, 1711–1717 (1965)
Hoshino, J., Simon, D., Kröger, H.: Identification of one of the l-serine dehydratase isoenzymes from rat liver as l-homoserine dehydratase. Biochem. biophys. Res. Commun. 44, 872–878 (1971)
Hoshino, J., Simon, D., Kröger, H.: Influence of monovalent cations on the activity of l-serine (l-threonine) dehydratase from rat liver. The control of threonine-serine activity ratio. Europ. J. Biochem. 27, 388–394 (1972)
Kagan, Z. S., Sinelnikova, E. M., Kretovich, W. L.: Biosynthetic l-threonine dehydratase of the meadow mushroom. Biokhimiya 34, 1279–1287 (1969)
Leitzmann, C., Bernlohr, R. W.: Threonine dehydratase of Bacillus licheniformis. I. Purification and properties. Biochim. biophys. Acta (Amst.) 151, 449–460 (1968)
Lessie, T. G., Whiteley, H. R.: Properties of threonine deaminase from a bacterium able to use threonine as sole source of carbon. J. Bact. 100, 878–889 (1969)
Maeba, P., Sanwal, B. D.: The allosteric threonine deaminase of Salmonella. Kinetic model for the native enzyme. Biochemistry 5, 525–536 (1966)
Monod, J., Changeux, J.-P., Jacob, F.: Allosteric proteins and cellular control systems. J. molec. Biol. 6, 306–329 (1963)
Nakagawa, H., Kimura, H.: The properties of crytalline serine dehydratase of rat liver. J. Biochem. (Tokyo) 66, 669–683 (1969)
Nishimura, J. S., Greenberg, D. M.: Purification and properties of l-threonine dehydrase of sheep liver. J. biol. Chem. 236, 2684–2691 (1961)
Raizada, M. K., Rao, V. K. M.: l-Threonine dehydratase activity of axenically grown Hartmannella culbertsoni. Arch. Mikrobiol. 84, 119–128 (1972)
Raskó, I., Alföldi, L.: Biosynthetic l-threonine deaminase as the origin of l-serine sensitivity of Escherichia coli. Europ. J. Biochem. 21, 424–427 (1971)
Sayre, F. W., Greenberg, D. M.: Purification and properties of serine and threonine dehydrases. J. biol. Chem. 220, 787–799 (1956)
Sharma, R. K., Mazumder, R.: Purification, properties, and feedback control of l-threonine dehydratase from spinach. J. biol. Chem. 245, 3008–3014 (1970)
Stanier, R. Y., Kunisawa, R., Mandel, M., Cohen-Bazire, G.: Properties and purification of unicellular blue-green algae (Order Chroococcales). Bact. Rev. 35, 171–205 (1971)
Thomas, D. A., Kuramitsu, H. K.: Biosynthetic l-threonine deaminase from Bacillus stearothermophilus. I. Catalytic and regulatory properties. Arch. Biochem. 145, 96–104 (1971)
Tomova, W. S., Kagan, Z. S., Kretovich, W. L.: l-threonine dehydratases from pea seedlings. Biokhimiya 33, 244–254 (1968)
Umbarger, H. E., Brown, B.: Threonine deamination in Escherichia coli. II. Evidence for two l-threonine deaminases. J. Bact. 73, 105–112 (1957)
Van Baalen, C.: Studies on marine blue-green algae. Bot. Mar. 4, 129–139 (1962)
Whiteley, H. R., Tahara, M.: Threonine deaminase of Clostridium tetanomorphum. I. Purification and properties. J. biol. Chem. 241, 4881–4889 (1966)
Wood, W. A., Gunsalus, I. C.: Serine and threonine deaminases of Escherichia coli: activators for a cell-free enzyme. J. biol. Chem. 181, 171–182 (1949)
Yanofsky, C., Reissig, J. L.: l-serine dehydrase of Neurospora. J. biol. Chem. 202, 567–577 (1953)
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Antia, N.J., Kripps, R.S. l-Threonine deaminase in marine planktonic algae. Archiv. Mikrobiol. 94, 29–46 (1973). https://doi.org/10.1007/BF00414076
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00414076