Summary
-
1.
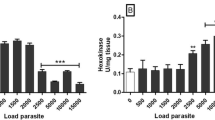
In the colourless acetate flagellate Polytomella caeca (Volvocales) 70–90% of the catalase activity and 60–90% of the uricase activity are particulate. After centrifugation of a crude particulate fraction on a sucrose gradient for 300 min at 60,000xg the distribution patterns of these microbodial marker enzymes overlap extensively those of cytochrome oxidase and succinic dehydrogenase marking the mitochondria. However, by centrifugation at 30,000 x g for 60 min a clear separation of the activity peaks of catalase and uricase from those of the mitochondrial enzymes can be achieved proving the existence of microbodies in Polymella caeca. After centrifugation at 60,000 x g for 15 hrs the microbodies were found around a density of 1.225 g/cm3.
-
2.
On an average more than 90% of the activity of the glyoxylate cycle enzymes isocitrate lyase and malate synthetase are found in the soluble fraction. But after washing the particulate fraction twice the specific activity of isocitrate lyase in this fraction drops only by half. After centrifugation (60,000 x g, 300 min) of a particulate fraction on a sucrose gradient a small peak of isocitrate lyase activity can be observed below the mitochondria fraction around a density of 1.24 g/cm3 where no peak of catalase or uricase activity occurs. Therefore, the small amount of particulate isocitrate lyase is not associated with the microbodies.
-
3.
30–40% of the acetyl-CoA synthetase are recovered in the particulate fraction. On sucrose gradients this enzyme shows distribution patterns very similar to cytochrome oxidase demonstrating its localization in the mitochondria. No peak or shoulder of the activity occurs in any other region of the gradient (except the top fraction) although an extramitochondrial activity of this enzyme has to be assumed.
-
4.
The experiments indicate that in Polytomella caeca the microbodies do not have a direct function in the metabolism of acetate.
Zusammenfassung
-
1.
In dem farblosen Acetatflagellaten Polytomella caeca (Volvocales) wurde unter Zugrundelegung von Katalase und Uricase als Leitenzymen das Vorkommen von Microbodies nachgewiesen. Nach Zentrifugation an einem Saccharose-Gradienten bei 60 000 x g für 15 Std sedimentieren die Microbodies bei einer Dichte von 1.225 g/cm3.
-
2.
Über 90% der Aktivitäten der Schlüsselenzyme des Glyoxylatcyclus wurden in der löslichen Fraktion eines Homogenats gefunden. Die geringe Aktivität der Isocitratlyase in der partikulären Fraktion wrwies sich als relativ fest gebunden, und nach zweimaligem Waschen der Partikelfraktion nahm die spezifische Aktivität nur um die Hälfte ab. An Saccharose-Gradienten trat nach Zentrifugation bei 60 000 x g für 300 min ein Gipfel der Isocitratlyaseaktivität bei der Dichte 1.24 g/cm3 auf.
-
3.
Partikuläre Acetyl-CoA-Synthetase konnte nur in den Mitochondrien lokalisiert werden.
-
4.
Für Polytomella caeca konnte eine unmittelbare Funktion der Microbodies im Acetatstoffwechsel nicht nachgewiesen werden.
Similar content being viewed by others
Literatur
Beevers, H.: Glyoxysomes of castor bean endosperm and their relation to gluconeogenesis. Ann. N. Y. Acad. Sci. 168, 209 (1969).
Bergmeyer, H.-U., Gawehn, K., Graßl, M.: Enzyme. Methoden der enzymatischen Analyse, Bd. I, S. 388–483, Hrsg.: H.-U. Bergmeyer, Weiheim/Bergstr.: Verlag Chemie 1970.
Breidenbach, R. W., Kahn, A., Beevers, H.: Characterization of glyoxysomes from castor bean endosperm. Plant Physiol. 43, 705–713 (1968).
Cooper, T. G., Beevers, H.: Mitochondria and glyoxysomes from castor bean endosperm. Enzyme constituents and catalytic capacity. J. biol. Chem. 244, 3507–3513 (1969).
Cooper, T. G., Beevers, H.: Fatty acid activation in glyoxysomes. Fed. Proc. 29, 868 Abs. (1970).
Dixon, G. H., Kornberg, H. L.: Malate synthetase from baker's yeast. Methods in enzymology, vol. V, pp. 633–637, ed. S. P. Colowick and N. O. Kaplan. New York: Academic Press 1962.
Duntze, W., Neumann, D., Gancedo, J. M., Atzpodien, W., Holzer, H.: Studies on the regulation and localization of the glyoxylate cycle enzymes in Saccharomyces cerevisiae. Europ. J. Biochem. 10, 83–89 (1969).
Frederick, S. E., Newcomb, E. H., Vigil, E. L., Wergin, W. P.: Fine-structural characterization of plant microbodies. Planta (Berl.) 81, 229–252 (1968).
Gerhardt, B., Beevers, H.: Influence of sucrose on protein determination by the Lowry procedure. Analyt. Biochem. 24, 337–339 (1968).
——: Developmental studies on glyoxysomes in Ricinus endosperm. J. Cell Biol. 44, 94–102 (1970).
—, Berger, Ch.: Microbodies und Diaminobenzidin-Reaktion in den Acetat-Flagellaten Polytomella caeca und Chlorogonium elongatum. Planta (Berl.) 100, 155–166 (1971).
Haigh, W. G., Beevers, H.: The glyoxylate cycle in Polytomella caeca. Arch. Biochem. 107, 152–157 (1964).
Harrop, L. C., Kornberg, H. L.: The role of isocitrate lyase in the metabolism of algae. Proc. roy. Soc. B 166, 11–29 (1966).
Hiatt, A. J.: Preparation and some properties of soluble succinic dehydrogenase from higher plants. Plant Physiol. 36, 552–557 (1961).
Hock, B., Beevers, H.: Development and decline of the glyoxylate cycle enzymes in watermelon seedling (Citrulus vulgaris Schrad.). Effects of dactinomycin and cycloheximide. Z. Pflanzenphysiol. 55, 405–414 (1966).
Kobr, M. J., Vanderhaeghe, F., Combépine, G.: Particulate enzymes of the glyoxylate cycle in Neurospora crassa Biochem. biophys. Res. Commun. 37, 640–645 (1969).
Lowenstein, J. M.: Citrate and the conversion of carbohydrate into fat. The metabolic roles of citrate, pp. 61–86, ed. T. W. Goodwin. New York: Academic Press 1968.
Lwoff, A., Ionesco, H., Gutman, A.: Synthèse et utilisation de l'amidon chez un flagellé sans chlorophylle incapable d'utiliser les sucress. Biochim. biophys. Acta. (Amst.) 4, 270–275 (1950).
Maehly, A. C., Chance, B.: The assay of catalases and peroxidases. Method of biochemical analysis, vol. I, pp. 361–424, ed. D. Glick, New York: Interscience Publishers Inc. 1954.
Müller, M., Hogg, J. F., de, Duve, Ch.: Distribution of tricarboxylic acid cycle enzymes and glyoxylate cycle enzymes between mitochondria and peroxisomes in Tetrahymena pyriformis. J. biol. Chem. 243, 5385–5395 (1968).
Nelson, E. B., Tolbert, N. E.: Glycolate dehydrogenase in green algae. Arch. Biochem. 141, 102–110 (1970).
Perlman, P. S., Mahler, H. R.: Intracellular localization of enzymes in yeast. Arch. Biochem. 136, 245–259 (1970).
Schneider, H.-J.: Porphyrin synthesis in isolated particles from tissue cultures of tobacco. Z. Naturforsch. (im Druck).
Seubert, W.: Buturyl-CoA and the CoA derivatives of the higher saturated fatty acids. Methods of enzymatic analysis, pp. 433–436, ed. H.-U. Bergmeyer. New York: Academic Press 1965.
Stadtman, E. R.: Preparation and assay of acyl coenzyme A and other thiol esters; use of hydroxylamine. Methods in enzymology, vol. III, pp. 931–941, ed. S. P. Colowick and N. O. Kaplan. New York: Academic Press 1957.
Szabo, A. S., Avers, Ch.-J.: Some aspects of regulation of peroxisomes and mitochondria in yeast. Ann. N. Y. Acad. Sci. 168, 302–312 (1969).
Takeda, Y., Suzuki, F., Inoue, H.: ATP citrate lyase (citrate-cleavage enzyme). Methods in enzymology, vol. XIII, pp. 153–160, ed. J. M. Lowenstein. New York: Academic Press (1969).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Gerhardt, B. Zur Lokalisation von Enzymen der Microbodies in Polytomella caeca . Archiv. Mikrobiol. 80, 205–218 (1971). https://doi.org/10.1007/BF00410122
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00410122