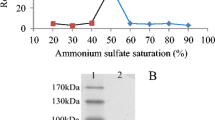
Abstract
An enzyme catalyzing the hydrolysis of purine nucleosides was found to occur in the extract of Azotobacter vinelandii, strain 0, and was highly purified by ammonium sulfate fractionation, DEAE-cellulose chromatography, hydroxylapatite chromatography and gel filtration on Sephadex G-150. A strict substrate specificity of the purified enzyme was shown with respect to the base components. The enzyme specifically attacked the nucleosides without amino groups in the purine moiety: inosine gave the maximum rate of hydrolysis and xanthosine was hydrolyzed to a lesser extent. The pH optimum of inosine hydrolysis was observed from pH 7 to 9, while xanthosine was hydrolyzed maximally at pH 7. The K m values of the enzyme for inosine were 0.65 and 0.85 mM at pH 7.1 and 9.0, respectively, and the value for xanthosine was 1.2 mM at pH 7.1.
Several nucleotides inhibited the enzyme: the phosphate portions of the nucleotides were suggested to be responsible for the inhibition by nucleotides. Although the inhibition of the enzyme by nucleotides was apparently non-competitive type with respect to inosine, allosteric (cooperative) binding of the substrate was suggested in the presence of the inhibitor. The physiological significance of the enzyme was discussed in connection with the degradation and salvage pathways of purine nucleotides.
Similar content being viewed by others
References
Achar, B. S., Vaidyanathan, C. S.: Purification and properties of uridine hydrolase from mung-bean (Phaseolus radiatus) seedlings. Arch. Biochem. Biophys. 119, 356–362 (1967)
Andrews, P.: Estimation of the molecular weights of proteins by Sephadex gel filtration. Biochem. J. 91, 222–233 (1964)
Carter, C. E.: Partial purification of a non-phosphorolytic uridine nucleosidase from yeast. J. Am. Chem. Soc. 73, 1508–1510 (1951)
Chen, P. S. Jr., Toribara, T. Y., Warner, H.: Microdetermination of phosphorus. Anal. Chem. 28, 1756–1758 (1956)
Clarke, J. T.: Simplified ‘disc’ (polyacrylamide gel) electrophoresis. Ann. N.Y. Acad. Sci. 121, 428–436 (1964)
Dewey, V. C., Kidder, G. W.: Partial purification and properties of a nucleoside hydrolase from Crithidia. Arch. Biochem. Biophys. 157, 380–387 (1973)
Dygert, S., Li, L. H., Florida, D., Thoma, J. A.: Determination of reducing sugar with improved precision. Anal. Biochem. 13, 367–374 (1965)
Friedmann, H. C., Harris, D. L.: The formation of α-glycosidic 5′-nucleotides by a single displacement trans-N-glycosidase. J. Biol. Chem. 240, 406–412 (1965)
Gardner, R., Kornberg, A.: Biochemical studies of bacterial sporulation and germination. V. Purine nucleoside phosphorylase of vegetative cells and spores of Bacillus cereus. J. Biol. Chem. 242, 2383–2388 (1967)
Guranowski, A., Schneider, Z.: Purification and characterization of adenosine nucleosidase from barley leaves. Biochim. Biophys. Acta 482, 145–158 (1977)
Henderson, J. F.: Nucleotide metabolism. An introduction. New York-London: Academic Press 1973
Heppel, L. A., Hilmoe, R. J.: Phosphorolysis and hydrolysis of purine ribosides by enzymes from yeast. J. Biol. Chem. 198, 683–694 (1952)
Kalckar, H. M.: Differential spectrophotometry of purine compounds by means of specific enzymes. I. Determination of hydroxypurine compounds. J. Biol. Chem. 167, 429–443 (1947)
Lampen, J. O., Wang, T. P.: The mechanism of action of Lactobacillus pentosus nucleosidase. J. Biol. Chem. 198, 385–395 (1952)
Lawrence, N. L.: The cleavage of adenosine by spores of Bacillus cereus. J. Bacteriol. 70, 577–582 (1955)
Lowry, O. H., Rosebrough, N. J., Farr, A. L., Randall, R. J.: Protein measurement with Folin-Phenol reagent. J. Biol. Chem. 193, 265–272 (1951)
MacNutt, W. S.: The enzymatically catalysed transfer of the deoxyribosyl group from one purine or pyrimidine to another. Biochem. J. 50, 384–397 (1952)
Mazelis, M., Creveling, R. K.: An adenosine hydrolase from Brussel sprouts. J. Biol. Chem. 238, 3358–3361 (1963)
Miller, G. W., Evans, H. J.: Nucleosidase from higher plants. Plant Physiol. 30, Suppl. 37 (1955)
Murray, A. W.: The biological significance of purine salvage. Ann. Rev. Biochem. 40, 811–826 (1971)
Parkes, R. E., Jr., Agarwal, R. P.: Purine nucleoside phosphorylase. In: The enzymes, Vol. 7, 3rd ed. (P. D. Boyer, ed.), pp. 483–514, New York: Academic Press 1972
Poulton, J. E., Butt, V. S.: Partial purification and properties of adenosine nucleosidase from leaves of spinach beet (Beta vulgaris L). Planta (Berl.) 131, 179–185 (1976)
Powell, J. F., Hunter, J. R.: Adenosine deaminase and ribosidase in spores of Bacillus cereus. Biochem. J. 62, 381–387 (1956)
Roberts, D. W. A.: The wheat leaf phosphatases. II. Pathways of hydrolysis of some nucleosides at pH 5.5. J. Biol. Chem. 222, 259–270 (1956)
Roush, A. H., Betz, R. F.: Purification and properties of trans-N-deoxyribosylase. J. Biol. Chem. 233, 261–266 (1958)
Schmidt, G., Walter, R. D., Königk, E. A purine nucleoside hydrolase from Trypanosoma gambiense. Purification and properties. Tropenmed. Parasit. 26, 19–26 (1975)
Schramm, V. L., Lazorik, F. C.: The pathway of adenylate catabolism in Azotobacter vinelandii. Evidence for adenosine monophosphate nucleosidase as the regulatory enzyme. J. Biol. Chem. 250, 1801–1808 (1975)
Serra, M. C., Falcone, G., Cercigmani, G., Ipata, P. L.: Some regulatory properties of purine nucleoside phosphorylase of Bacillus cereus. FEBS Lett. 18, 335–338 (1971)
Takagi, Y., Horecker, B. L.: Purification and properties of a bacterial riboside hydrolase. J. Biol. Chem. 225, 77–86 (1957)
Tarr, H. L. A.: Fish muscle riboside hydrolase. Biochem. J. 59, 386–391 (1955)
Terada, M., Tatibana, M., Hayaishi, O.: Purification and properties of nucleoside hydrolase from Pseudomonas fluorescens. J. Biol. Chem. 242, 5578–5585 (1967)
Yoshino, M., Ogasawara, N., Suzuki, N., Kotake, Y.: Regulation of AMP nucleosidase in Azotobacter vinelandii. Biochim. Biophys. Acta. 146, 620–622 (1967)
Yoshino, M.: AMP nucleosidase from Azotobacter vinelandii. I. Purification and properties. J. Biochem. 68, 321–329 (1970)
Yoshino, M., Ogasawara, N.: AMP nucleosidase from Azotobacter vinelandii. III. Kinetics of allosteric interactions. J. Biochem. 72, 223–233 (1972)
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Yoshino, M., Tsukada, T. & Tsushima, K. Inosine nucleosidase from Azotobacter vinelandii . Arch. Microbiol. 119, 59–64 (1978). https://doi.org/10.1007/BF00407928
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00407928