Abstract
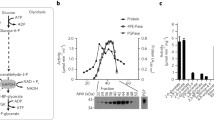
Experimental conditions have been elaborated to test for reversibility of the malate dehydrogenase inactivation (E.C.1.1.1.37) after addition of glucose to derepressed yeast cells. Malate dehydrogenase inactivation was shown to be irreversible at all stages of inactivation. In contrast fructose-1,6-bisphosphatase inactivation (E.C.3.1.11) remained reversible for at least 30 min after addition of glucose.
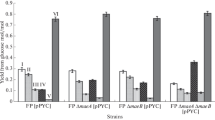
Rapid reversible inactivation of fructose-1,6-bisphosphatase and irreversible inactivation of malate dehydrogenase were additionally investigated in glycolytic block mutants. Normal inactivation kinetics were observed in mutants without catalytic activity of phosphoglucoseisomerase (E.C.5.3.1.9), phosphofructokinase (E.C.2.7.1.11), triosephosphate isomerase (E.C.5.3.1.1) and phosphoglycerate kinase (E.C.2.7.2.3). Hence, neither type of inactivation depended on the accumulation of any glucose metabolite beyond glucose-6-phosphate. Under anaerobic conditions irreversible inactivation was completely abolished in glycolytic block mutants. In contrast rapid reversible inactivation was independent of energy provided by respiration or fermentation.
Reversibility of fructose-1,6-bisphosphatase inactivation was tested under conditions which prevented irreversible malate dehydrogenase inactivation. In these experiments, fructose-1,6-bisphosphatase inactivation remained reversible for at least 120 min, whereas reversibility was normally restricted to about 30 min. This indicated a common mechanism between the irreversible part of fructose-1,6-bisphosphatase inactivation and irreversible malate dehydrogenase inactivation.
Similar content being viewed by others
References
Ciriacy M (1975) Genetics of alcohol dehydrogenase in Saccharomyces cerevisiae. Isolation and genetic analysis of mutants. Mutat Res 29:315–325
Ciriacy M, Breitenbach I (1979) Physiological effect of seven different blocks in glycolysis in Saccharomyces cerevisiae. J Bacteriol 139:152–160
Clifton D, Fraenkel DG (1981) The ger (glycolysis regulation) mutation of Saccharomyces cerevisiae. J Biol Chem 256:13074–13078
Clifton D, Weinstock SB, Fraenkel DG (1978) Glycolysis mutants in Saccharomyces cerevisiae. Genetics 88:1–11
Duntze W, Neumann D, Gancedo JM, Atzpodien W, Holzer H (1969) Studies on the regulation and localisation of the glyoxylate cycle enzymes in Saccharomyces cerevisiae. Eur J Biochem 10:83–89
Entian K-D, Zimmermann FK (1980) Glycolytic enzymes and intermediates in carbon catabolite repression mutants of Saccharomyces cerevisiae. Mol Gen Genet 177:345–350
Entian K-D, Zimmermann FK, Scheel J (1977) A partial defect in carbon catabolite repression in mutants of Saccharomyces cerevisiae with reduced hexose phosphorylation. Mol Gen Genet 156:99–105
Fröhlich K-U, Entian K-D (1982) Regulation of gluconeogenesis in the yeast Saccharomyces cerevisiae. Evidence for conversion of enolase isoenzymes. FEBS Lett 139:164–166
Funayama S, Gancedo JM, Gancedo C (1980) Turnover of yeast fructose-1,6-bisphosphatase in different metabolic conditions. Eur J Biochem 109:61–66
Gancedo C (1971) Inactivation of fructose-1,6-bisphosphatase by glucose in yeast. J Bacteriol 107:401–405
Gancedo JM, Gancedo C (1971) Fructose-1,6-diphosphatase, phosphofructo-kinase and glucose-6-phosphate dehydrogenase from fermenting and non-fermenting yeast. Arch Microbiol 76:132–138
Gancedo JM, Gancedo C (1979) Inactivation of gluconeogenic enzymes in glycolytic block mutants of Saccharomyces cerevisiae. Eur J Biochem 101:455–460
Gancedo C, Salas ML, Giner A, Sols A (1965) Reciprocal effects of carbon sources on the level of an AMP-sensitive fructose-1,6-diphosphatase and phosphofructokinase in yeast. Biochem Biophys Res Commun 20:15–20
Gancedo C, Schwerzmann N (1976) Inactivation by glucose of phosphoenol-pyruvate carboxykinase from Saccharomyces cerevisiae. Arch Microbiol 109:221–225
Gascon S, Neumann NP, Lampen JO (1968) Comparative study of the properties of the purified internal and external invertases from yeast. J Biol Chem 243:1573–1577
Grossmann MK, Zimmermann FK (1979) The structural genes of internal invertases in Saccharomyces cerevisiae. Mol Gen Genet 175:223–229
Haarasilta S, Oura E (1975) On the activity and regulation of anaplerotic and gluconeogenetic enzymes during the growth process of baker's yeast. The biphasic growth. Eur J Biochem 52:1–7
Hägele E, Neeff J, Mecke D (1978) The malate dehydrogenase isoenzymes of Saccharomyces cerevisiae. Purification, characterization and studies on their regulation. Eur J Biochem 83:67–76
Holzer H (1976) Catabolite inactivation in yeast. Trends Biochem Sci 1:178–181
Lenz A-G, Holzer H (1980) Rapid reversible inactivation of fructose-1,6-bisphosphatase in Saccharomyces cerevisiae by glucose
Magasanik B (1961) Catabolite repression. Cold Spring Harbor Symp Quant Biol 26:249–256
Müller D, Holzer H (1981) Regulation of fructose-1,6-bisphosphatase in yeast by phosphorylation/dephosphorylation. Biochem Biophys Res Commun 103:926–933
Müller M, Müller H, Holzer H (1981) Immunochemical studies on catabolite inactivation of phosphoenolpyruvate carboxykinase in Saccharomyces cerevisiae. J Biol Chem 256:723–727
Polakis ES, Bartley W (1965) Changes in the enzyme activities of Saccharomyces cerevisiae during aerobic growth on different carbon sources. Biochem J 97:284–297
Polakis ES, Bartley W, Meek GA (1965) Changes in the activities of respiratory enzymes during the aerobic growth of yeast on different carbon sources. Biochem J 97:298–302
Tortora P, Birtel M, Lenz A-G, Holzer H (1981) Glucose-dependent inactivation of fructose-1,6-bisphosphatase in yeast. Biochem Biophys Res Commun 100:688–695
Wijk R van, Ouwehand J, Bos T van den, Königsberger VV (1969) Induction and catabolite repression of alpha-glucosidase synthesis in protoplasts of Saccharomyces carlsbergensis. Biochim Biophys Acta 186:178–191
Witt J, Kronau R, Holzer H (1966a) Isoenzyme der Malatdehydrogenase und ihre Regulation in Saccharomyces cerevisiae. Biochim Biophys Acta 128:63–73
Witt I, Kronau R, Holzer H (1966b) Repression von Alkoholdehydrogenase, Malatdehydrogenase, Isocitratlyase und Malatsynthase in Hefe durch Glucose. Biochim Biophys Acta 118:522–237
Wolfe RG, Neilands JB (1956) Some molecular and kinetic properties of heart malic dehydrogenase. J Biol Chem 221:61–69
Zamenhoff S (1957) Preparation and assay of desoxyribonucleic acids from animal tissue. In: Colowick SP, Kaplan NO (eds) Methods in enzymology, vol III. Academic Press, London New York, pp 696–704
Zimmermann FK, Eaton NR (1974) Genetics of induction and catabolite repression of maltase synthesis in Saccharomyces cerevisiae. Mol Gen Genet 134:261–272
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Entian, KD., Dröll, L. & Mecke, D. Studies on rapid reversible and non-reversible inactivation of fructose-1,6-bisphosphatase and malate dehydrogenase in wild-type and glycolytic block mutants of Saccharomyces cerevisiae . Arch. Microbiol. 134, 187–192 (1983). https://doi.org/10.1007/BF00407756
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00407756