Abstract
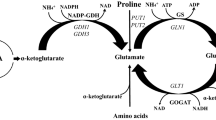
NAD-specific glutamate dehydrogenase (GDH-B)1 was induced in a wild-type strain derived of α-Σ 1278b by α-amino acids, the nitrogen of which according to known degradative pathways is transferred to 2-oxoglutarate. A recessive mutant (gdhB) devoid of GDH-B activity grew more slowly than the wild type if one of these amino acids was the sole source of nitrogen. Addition of ammonium chloride, glutamine, asparagine or serine to growth media with inducing α-amino acids as the main nitrogen source increased the growth rate of the gdhB mutant to the wild-type level and repressed GDH-B synthesis in the wild type. Arginine, urea and allantoin similarly increased the growth rate of the gdhB mutant and repressed GDH-B synthesis in the presence of glutamate, but not in the presence of aspartate, alanine or proline as the main nitrogen source. These observations are consistent with the view that GDH-B in vivo deaminates glutamate. Ammonium ions are required for the biosynthesis of glutamine, asparagine, arginine, histidine and purine and pyrimidine bases. Aspartate and alanine apparently are more potent inducers of GDH-B than glutamate.
Anabolic NADP-specific glutamate dehydrogenase (GDH-A) can not fulfil the function of GDH-B in the gdhB mutant. This is concluded from the equal growth rates in glutamate, aspartate and proline media as observed with a gdhB mutant and with a gdhA, gdhB double mutant in which both glutamate dehydrogenases are lacking. The double mutant showed an anomalous growth behaviour, growth rates on several nitrogen sources being unexpectedly low.
Similar content being viewed by others
Abbreviations
- GDH-A:
-
NADP-specific glutamate dehydrogenase [l-glutamate
- NADP+ :
-
oxido-reductase (deaminating), EC 1.4.1.4]
- gdhA :
-
genotype associated with GDH-A deficiency
- GDH-B:
-
NAD-specific glutamate dehydrogenase, [L-glutamate NAD+ oxido-reductase (deaminating), EC 1.4.1.2]
- gdhB :
-
genotype associated with GDH-B deficiency
- gdhCR :
-
genotype associated with derepressed GDH-B synthesis
- μ:
-
specific growth rate (h-1)
- x:
-
cell density
- t:
-
time (h)
References
Bernhardt, W., Panten, K. und Holzer, H. 1965. Gedämpftes Oscillieren der Synthesegeschwindigkeit von DPN-abhängiger Glutamatdehydrogenase in Hefezellen. — Biochim. Biophys. Acta 99: 531–539.
Cennamo, C., Boll, M. und Holzer, H. 1964. Ueber Threonindehydratase aus Saccharomyces cerevisiae. — Biochem. Z. 340: 125–145.
Doherty, D. 1970. l-Glutamate dehydrogenases (yeast). p. 850–856. In H. Tabor and C. W. Tabor, (eds), Methods in Enzymology. Vol. 17. — Academic Press Inc., New York and London.
Dubois, E. and Grenson, M. 1974. Absence of involvement of glutamine synthetase and of NAD-linked glutamate dehydrogenase in the nitrogen catabolite repression of arginase and other enzymes in Saccharomyces cerevisiae. — Biochem. Biophys. Res. Commun. 60: 150–157.
Grenson, M., Dubois, E., Piotrowska, M., Drillien, R. and Aigle, M. 1974. Ammonia assimilation in Saccharomyces cerevisiae as mediated by the two glutamate dehydrogenases. — Molec. Gen. Genet. 128: 73–85.
Grenson, M., and Hou, C. 1972. Ammonia inhibition of the general amino acid permease and its suppression in NADPH-specific glutamate dehydrogenase-less mutants in Saccharomyces cerevisiae. — Biochem. Biophys. Res. Commun. 48: 749–756.
Hemmings, B. A. and Sims, A. P. 1977. The regulation of glutamate metabolism in Candida utilis. Evidence for two interconvertible forms of NAD-dependent glutamate dehydrogenase. — Eur. J. Biochem. 80: 143–151.
Kinghorn, J. R. and Pateman, J. A. 1976. Mutants of Aspergillus nidulans lacking nicotinamide adenine dinucleotide-specific glutamate dehydrogenase. — J. Bacteriol. 125: 42–47.
Middelhoven, W. J. 1964. The pathway of arginine brakdown in Saccharomyces cerevisiae. —Biochim. Biophys. Acta 93: 650–652.
Middelhoven, W. J. 1969. Enzyme repression in the arginine pathway of Saccharomyces cerevisiae. —Antonie van Leeuwenhoek 35: 215–226.
Middelhoven, W. J. 1977. Isolation and characterization of methylammonium-resistant mutants of Saccharomyces cerevisiae with relieved nitrogen metabolite repression of allantoinase, arginase and ornithine transaminase synthesis. — J. gen. Microbiol. 100: 257–269.
Middelhoven, W. J., Broekhuizen, B. and Van Eijk, J. 1976. The detection of yeast mutants unable to utilize nitrogenous substances as the sole nitrogen source with the dye Phloxine B. — J. Bacteriol. 128: 851–852.
Roon, R. J. and Even, H. L. 1973. Regulation of nicotinamide adenine dinucleotide and nicotinamide adenine dinucleotide phosphate-dependent glutamate dehydrogenase of Saccharomyces cerevisiae. — J. Bacteriol. 116: 367–372.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Middelhoven, W.J., van Eijk, J., van Renesse, R. et al. A mutant of Saccharomyces cerevisiae lacking catabolic NAD-specific glutamate dehydrogenase Growth characteristics of the mutant and regulation of enzyme synthesis in the wild-type strain. Antonie van Leeuwenhoek 44, 311–320 (1978). https://doi.org/10.1007/BF00394308
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00394308