Abstract
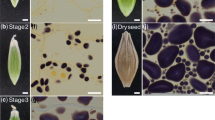
The structure of plastids in the root cap of cress and maize was studied by low- and high-voltage electron microscopy after staining their membranes with a mixture of zinc iodide and osmium tetroxide. In plastids of both species electron-opaque membranes were found in the plastid interior while membranes of lesser electron-opacity comprised the outer envelope and vesicles and cisternae underlying it. Electron-opaque tubules, often in groups attached to the inner membrane of the amyloplast envelope, were found in cress but not in maize. The internal, less-opaque membranes were often found associated with the starch grains. No specific association could be seen between amyloplasts and endoplasmic reticulum (ER); their surfaces showed no regular contact or connexion, though the amyloplasts clearly indented the underlying ER. The ER in statocytes was predominantly tubular in cress but predominantly cisternal in maize.
Similar content being viewed by others
Abbreviations
- ER:
-
endoplasmic reticulum
- ZIO:
-
zinc iodideosmium tetroxide
References
Barlow, P.W. (1975) The root cap. In: The development and function of roots, pp. 21–54, Torrey, J.G., Clarkson, D.T., eds. Academic Press, London New York San Francisco
Brossard-Chriqui, D., Iskander, S. (1980) Particularités ultrastructurales de l'amylogenèse provoquée in vitro dans les explants foliaires du Datura innoxia Mill. J. Ultrastruct. Res. 72, 264–271
Brossard-Chriqui, D., Iskander, S. (1982) Ultrastructural changes of cytomembranes during the first hours of the in vitro culture of Datura innoxia Mill, leaf explants. Relations to sucrose uptake. Protoplasma 112, 217–225
Dauwalder, M., Whaley, W.G. (1973) Staining of cells of Zea mays root apices with osmium-zinc iodide and osmium impregnation techniques. J. Ultrastruct. Res. 45, 279–296
Dexheimer, J., Muller, J., Riedacker, A. (1982) Etude ultrastructurale des coiffes des racines de chêne (Quercus robur). I. Les pivots. Can. J. Bot. 60, 620–629
Gilloteaux, J., Naud, J. (1979) The zinc iodide-osmium tetroxide staining-fixative of Maillet. Nature of the precipitate studied by X-ray microanalysis and detection of Ca2+-affinity subcellular sites in a tonic smooth muscle. Histochemistry 63, 227–243
Hawes, C.R. (1981) Applications of high voltage electron microscopy to botanical ultrastructure. Micron 12, 227–267
Hawes, C.R., Juniper, B.E., Horne, J.C. (1981) Low and high voltage electron microscopy of mitosis and cytokinesis in maize roots. Planta 153, 397–407
Huber, W., de Fekete, M.A.R., Ziegler, H. (1973) Enzyme des Stärkeumsatzes in den Wurzelhaubenzellen von Zea mays L. Planta 112, 343–356
Iversen, T.-H. (1974) The role of statoliths, auxin transport and auxin metabolism in root geotropism. K. Norske Vidensk. Selsk. Mus. Miscell 15, 1–216
Iversen, T.-H., Rommelhoff, A. (1978) The starch statolith hypothesis and the interaction of amyloplasts and endoplasmic reticulum in root geotropism. J. Exp. Bot. 29, 1319–1328
Juniper, B.E. (1977) The perception of gravity by a plant. Proc. R. Soc. London Ser. B 199, 537–550
Macdonald, F.D., ap Rees, T. (1983) Enzymic properties of amyloplasts from suspension cultures of soybean. Biochim. Biophys. Acta 755, 81–89
Marty, F. (1980) High voltage electron microscopy of membrane interaction in wheat. J. Histochem. Cytochem. 28, 1129–1132
Moore, R., McLelen, C.E. (1983) A morphometric analysis of cellular differentiation in the root cap of Zea mays. Am. J. Bot. 70, 611–617
Ohad, I., Friedberg, I., Ne'eman, Z., Schramm, M. (1971) Biogenesis and degradation of starch. I. The fate of the amyloplast membranes during maturation and storage of potato tubers. Plant Physiol. 47, 465–477
Perbal, G. (1978) The mechanism of geoperception in lentil roots. J. Exp. Bot. 29, 631–638
Poux, N. (1973) Observations en microscope électronique de cellules végétales impregnées par l'osmium. C.R. Acad. Sci. Paris Ser. D 276, 2163–2166
Poux, N. (1981) Analyse de la cellule végétale par microscopie électronique à haut voltage. Bull. Soc. Bot. Fr. 128, 7–18
Rivier, L., Pilet, P.-E. (1974) Indolyl-3-acetic acid in cap and apex of maize roots: identification and quantification by mass fragmentography. Planta 120, 107–112
Rodriguez-García, M.I., Sievers, A. (1977) Membrane contacts of the endoplasmic reticulum with plastids and with plasmalemma in the endothecium ofScilla non-scripta. Cytobiologie 15, 85–95
Schilling, N., Dittrich, P. (1979) Interaction of hydrolytic and phosphorolytic enzymes of starch metabolism in Kalanchoë daigremontiana. Planta 147, 210–215
Schmitz, U. (1977) Ultrastruktur und Geoperception in der Kalyptra von Seitenwurzeln. Diss. Bot. 37, 1–103
Schneider, E.M., Becker, J.-U., Volkmann, D. (1981) Biochemical properties of potato phosphorylase change with its intracellular localization as revealed by immunological methods. Planta 151,124–134
Sievers, A., Volkmann, D. (1977) Ultrastructure of gravity-perceiving cells in plant roots. Proc. R. Soc. London Ser. B 199, 525–536
Sobczyk, U., Szweykowska, A. (1975) The effect of 3-indolylacetic acid on the accumulation of starch in the root tissue of Cichorium intybus L. cultured in vitro. Acta Soc. Bot. Pol. 44, 297–303
Spurr, A.R. (1969) A low viscosity epoxy resin embedding medium for electron microscopy. J. Ultrastruct. Res. 26, 31–43
Volkmann, D. (1974) Amyloplasten und Endomembranen.Das Geoperzeptionssystem der Primärwurzel. Protoplasma 79, 159–183
Whatley, J.M. (1983) Plastids in the roots of Phaseolus vulgaris. New Phytol. 94, 381–391
Whatley, J.M., Hawes, C.R., Horne, J.C., Kerr, J.D.A. (1982) The establishment of the plastid thylakoid system. New Phytol. 90,619–629
Woźny, A., Gwóźdź, E., Szweykowska, A. (1973) The effect of 3-indolylacetic acid on the differentiation of plastids in callus culture of Cichorium intybus L. Protoplasma 76, 109–114
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Barlow, P.W., Hawes, C.R. & Horne, J.C. Structure of amyloplasts and endoplasmic reticulum in the root caps of Lepidium sativum and Zea mays observed after selective membrane staining and by high-voltage electron microscopy. Planta 160, 363–371 (1984). https://doi.org/10.1007/BF00393418
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00393418