Abstract
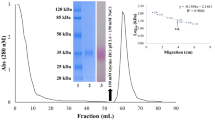
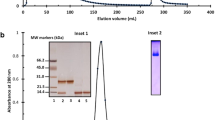
Concanavalin A (Con A) is a tetrameric lectin which is synthesized in the cotyledons of developing jack-bean (Canavalia ensiformis (L.) D.C.) seeds and accumulates in the protein bodies of storage-parenchyma cells. The polypeptides of Con A have a molecular weight of 27000 and a relative molecular mass (Mr) of 30000 when analyzed by gel electrophoresis on denaturing polyacrylamide gels. In-vitro translation of RNA isolated from immature jack-bean cotyledons shows that Con A is synthesized as a polypeptide with Mr 34000. In-vivo pulse labeling of cotyledons with radioactive amino acids or glucosamine also resulted in the formation of a 34000-Mr polypeptide. In-vivo labeling with radioactive amino acids in the presence of tunicamycin yielded an additional polypeptide of 32000 Mr. Together these results indicate that Con A is cotranslationally processed by the removal of a signal sequence and the addition of an oligosaccharide side chain of corresponding size. Analysis of the structure of the oligogosaccharide side chain was accomplished through glycosidase digestion of glycopeptides isolated from [3H]glucosamine-labeled Con A. Incubation of the labeled glycopeptides with endoglycosidase H, α-mannosidase or β-N-acetylglucosaminidase, followed by gel filtration, allowed us to deduce that the oligosaccharide side chain of pro-Con A is a high-mannose oligosaccharide. Pulse-chase experiments with labeled amino acids are consistent with the interpretation that the glycosylated precursor of Con A is processed to mature Con A (Mr=30000). The 4000 decrease in Mr is interpreted to result from the removal of a small glycopeptide. The implications of the conversion of a glycoprotein pro-Con A to mature Con A are discussed in the context of the unique circular permutation of the primary structure of Con A.
Similar content being viewed by others
Abbreviations
- Con A:
-
concanavalin A
- Glc:
-
glucose
- GlcNAc:
-
N-acetylglucosamine
- IgG:
-
immunoglobulin G
- Man:
-
mannose
- Mr :
-
relative molecular mass
- SDS-PAGE:
-
sodium dodecylsulfate-polyacrylamide gel electrophoresis
References
Becker, J.W., Cunningham, B.A., Hemperley, J.J. (1983) Structural subclasses of lectins from leguminous plants. In: Chemical taxonomy, molecular biology, and function of plant lectins, pp. 31–45, Goldstein, I.J., Etzler, M.E. eds. Alan R. Liss, New York
Bittiger, H., Schnebli, H.P. (1976) Concanavalin as a tool. John Wiley & Sons, London, UK
Bollini, R., Chrispeels, M.J. (1979) The rough endoplasmic reticulum is the site of reserve-protein synthesis in developing Phaseolus vulgaris cotyledons. Planta 146, 487–501
Bollini, R., Vitale, A., Chrispeels, M.J. (1983) In vivo and in vitro processing of seed reserve protein in the endoplasmic reticulum: Evidence for two glycosylation steps. J. Cell Biol. 96, 999–1007
Bonner, W.M., Laskey, R.A.(1974) A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur. J. Biochem. 46, 83–88
Carrington, D.M., Auffret, A., Hanke, D.E. (1985) Polypeptide ligation occurs during post-translational modification of concanavalin A. Nature 313, 64–67
Chrispeels, M.J. (1983) The Golgi apparatus mediates the transport of phytochemagglutinin to the protein bodies in bean cotyledons. Planta 158, 140–151
Chrispeels, M.J. (1984) Biosynthesis processing and transport of storage proteins and lectins in cotyledons of developing legume seeds. Phil. Trans. R. Soc. London Ser.B 304, 309–322
Chrispeels, M.J., Higgins, T.J.V., Craig, S., Spencer, D. (1982) Role of the endoplasmic reticulum in the synthesis if reserve proteins and the kinetics of their transport to protein bodies in developing pea cotyledons. J. Cell Biol. 93, 5–14
Cunningham, B.A., Hemperly, J.J., Hopp, T.P., Edelman, G.M. (1979) Favin versus concanavalin A: circularly permuted amino acid sequences. Proc. Natl. Acad. Sci. USA 76, 3218–3222
Cunningham, B.A., Wang, J.L., Waxdal, M.J., Edelman, G.M. (1975) The covalent and three dimensional structure of concanavalin A. II. Amino acid sequence of cyanogen bromide fragment F3. J. Biol. Chem. 250, 1503–1512
Dorland, L., Van Halbeek, H., Vliegenthart, J.F.G., Lis, H., Sharon, N. (1981) Primary structure of the carbohydrate chain of soybean agglutinin. J. Biol. Chem. 256, 7708–7711
Edmundson, A.B., Ely, K.R., Sly, D.A., Westholm, F.A., Powers, D.A., Liener, I.E. (1971) Isolation and characterization of concanavalin A polypeptide chains. Biochemistry 10, 3554–3559
Foriers, A., De Neve, R., Kanarek, L., Strosberg, A.D. (1978) Common ancestor for concanavalin A and lentil lectin. Proc. Natl. Acad. Sci. USA 75, 1136–1139
Foriers, A., Lebrun, E., Van Rapenbusch, R., De Neve, R., Strosberg, A.D. (1981) The structure of the lentil (Lens culinaris) lectin. J. Biol. Chem. 256, 5550–5560
Goldstein, I.J., Hayes, C.E. (1978) The lectins: Carbohydrate-binding proteins of plants and animals. Adv. Carbohydr. Chem. Biochem. 35, 127–340
Hemperley, J.J., Mostov, K.E., Cunningham, B.A. (1982) In vitro translation and processing of a precursor form of Favin, a lectin from Vicia faba. J. Biol. Chem. 257, 7903–7909
Herbert, E., Uhler, M. (1982) Biosynthesis of polyprotein precursors to regulatory peptides. Cell 30, 1–2
Herman, E.M., Shannon, L.M. (1984a) Immunocytochemical localization of concanavalin A in developing jack-bean cotyledons. Planta 161, 97–104
Herman, E.M., Shannon, L.M. (1984b) Immunocytochemical evidence for the involvement of Golgi apparatus in the deposition of seed lectin of Bauhinia purpurea (Leguminosae). Protoplasma 121, 163–170
Higgins, T.J.V. (1984) Synthesis and regulation of major proteins in seeds. Annu. Rev. Plant Physiol. 35, 191–221
Higgins, T.J.V., Chandler, P.M., Zurawski, G., Button, S.C., Spencer, D. (1983a) The biosynthesis and primary structure of pea seed lectin. J. Biol. Chem. 258, 9544–9549
Higgings, T.J.V., Chrispeels, M.J., Chandler, P.M., Spencer, D. (1983b) Intracellular sites of synthesis and processing of lectin in developing pea cotyledons. J. Biol. Chem. 258, 9950–9552
Julius, D., Schekman, R., Thorner, J. (1984) Glycosylation and processing of prepro-α-factor through the yeast secretory pathway. Cell 36, 309–316
Laemmli, U.K., Favre, M. (1973) Maturation of the head of bacteriophage T4. J. Mol. Biol. 80, 575–599
Langridge, P., Pintor, J.A., Felx, G. (1982) Zein precursor mRNAs from maize endosperm. Mol. Gen. Genet. 187, 432–438
Marcus, S.E., Burgess, J., Maycox, P.R., Bowles, D.J. (1984) A study of maturation events in jack-beans (Canavalia ensiformis). Biochem. J. 222, 265–268
Millerd, A., Spencer, D., Dudman, W.F., Stiller, M. (1975) Growth of immature pea cotyledons in culture. Aust. J. Physiol. 2, 51–59
Misaki, A., Goldstein, I.J. (1977) Glycosyl moiety of the lima bean lectin. J. Biol. Chem. 252, 6995–6999
Spencer, D., Higgins, T.J.V., Button, S.C., Davey, R.A. (1980) Pulse-labeling studies on protein synthesis in developing pea seeds and evidence of a precursor form of legumin small subunit. Plant Physiol. 66, 510–515
Staswick, P., Chrispeels, M.J. (1984) Expression of lectin genes during seed development in normal and phytohemagglutinin-deficient cultivars of Phaseolus vulgaris. J. Mol. Appl. Genet. 2, 525–535
Strosberg, A.D., Lauwereys, M., Foriers, A. (1983) Molecular evolution of legume lectins. In: Chemical taxonomy, molecular biology, and function of plant lectins, pp. 7–20, Goldstein, I.J., Etzler, M.E., eds Alan R. Liss, New York
Tarentino, A.L., Maley, F. (1974) Purification and properties of an endo-β-N-acetylglucosaminidase from Streptomyces griseus. J. Biol. Chem. 249, 811–817
Ternynck, T., Avrameas, S. (1972) Polyacrylamide-protein immunoadsorbents prepared with glutaraldehyde. FEBS Lett. 23, 24–28
Vitale, A., Ceriotti, A., Bollini, R., Chrispeels, M.J. (1984a) Biosynthesis and processing of phytohemagglutin in developing bean cotyledons. Eur. J. Biochem. 141, 97–104
Vitale, A., Chrispeels, M.J. (1984) Transient N-acetylglucosamine in the biosynthesis of phytohemagglutinin: Attachment in the Golgi apparatus and removal in protein bodies. J. Cell Biol. 99, 133–140
Vitale, A., Warner, T.G., Chrispeels, M.J. (1984b) Phaseolus vulgaris phytohemagglutinin contains high mannose and modified oligosaccharide chains. Planta 160, 256–263
Vodkin, L.O. (1981) Isolation and characterization of messenger RNAs for seed lectin and Kunitz trypsin inhibitor in soybeans. Plant Physiol. 68, 766–771
Wang, J.L., Cunningham, B.A., Edelman, G.M. (1971) Unusual fragments in the subunit structure of concanavalin A. Proc. Natl. Acad. Sci. USA 68, 1130–1134
Wang, J.L., Cunningham, B.A., Waxdal, M.J., Edelman, G. (1975) The covalent and three dimensional structure of concanavalin A. I. Amino acid sequence of cyanogen bromide fragments F1 and F2. J. Biol. Chem. 250, 1490–1502
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Herman, E.M., Shannon, L.M. & Chrispeels, M.J. Concanavalin A is synthesized as a glycoprotein precursor. Planta 165, 23–29 (1985). https://doi.org/10.1007/BF00392207
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00392207