Summary
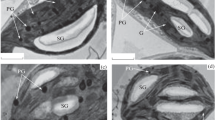
The enzyme patterns in sunflower cotyledons indicate that the glyoxysomal function of microbodies is replaced by the peroxisomal function of these organelles during the transition from fat degradation to photosynthesis. The separation of the microbody population into glyoxysomes and peroxisomes during this transition period is reported. The mean difference in density between the activity peaks of glyoxysomal and peroxisomal marker enzymes on a sucrose gradient was calculated to be 0.007±0.004 g/cm3 and turned out to be significant (t=7.8>4.04=t 5;0.01). The activity peak of catalase coincides with that of isocitrate lyase in early stages of development, but shifts to the activity peak of peroxisomal marker enzymes during the transition period. No isozymes of the catalase could be detected by gel electrophoresis in the microbodies with the two different functions.
During the rise of the peroxisomal marker enzymes no synthesis of the common microbody marker, catalase, could be demonstrated using the inhibitor allylisopropylacetamide. Using D2) for density labeling of newly-formed catalase, no difference is observed between the density of catalase from cotyledons grown on 99.8% D2O during the transition period and the density of enzyme from cotyledons grown on H2O. The activity of particulate glycolate oxidase is reduced 30–50% by allylisopropylacetamide, but is not affected by D2O. The chlorophyll formation in the cotyledons is strongly inhibited by both substances.
Similar content being viewed by others
Literatur
Anstine, W., Jacobsen, J. V., Scandalios, J. G., Varner, J. E.: Deuterium oxide as density label of peroxidases in germinating barley embryos. Plant Physiol. 45, 148–152 (1970).
Arnon, D. J.: Copper enzymes in isolated chloroplasts. Polyphenol oxidase in Beta vulgaris. Plant Physiol. 24, 1–15 (1949).
Baker, A. L., Tolbert, N. E.: Glycolate oxidase. Methods in enzymology, vol. IX, p. 338–342 (Wood, W. A., ed.). New York: Academic Press 1966.
Berger, Ch., Gerhardt, B.: Charakterisierung der Microbodies aus Spadix-Appendices von Arum maculatum L. and Sauromatum guttatum Schott. Planta (Berl.) 96, 326–338 (1971).
Bruner, R., Vinograd, J.: The evaluation of standard sedimentation coefficients of sodium RNA and sodium DNA from sedimentation velocity data in concentrated NaCl and CsCl solutions. Biochim. biophys. Acta (Amst.) 108, 18–29 (1965).
Drumm, H., Falk, H., Möller, J., Mohr, H.: The development of catalase in the mustard seedling. Cytobiol. 2, 335–340 (1970).
Feierabend, J., Beevers, H.: Developmental studies on microbodies in wheat leaves. II. Ontogeny of particulate enzyme associations. Plant Physiol. 49, 33–39 (1972).
Filner, P., Varner, J. E.: A test for de novo synthesis of enzymes: density labeling with H2 18O of barley-amylase induced by gibberellic acid. Proc. nat. Acad. Sci. (Wash.) 58, 1520–1526 (1967).
Gerhardt, B.: Zur Lokalisation von Enzymen der Microbodies in Polytomella caeca. Arch. Mikrobiol. 80, 205–218 (1971).
Gerhardt, B., Beevers, H.: Developmental studies on glyoxysomes in Ricinus endosperm. J. Cell Biol. 44, 94–102 (1970).
Gruber, P. J., Trelease, R. N., Becker, W. M., Newcomb, E. H.: A correlative ultrastructural and enzymatic study of cotyledonary microbodies following germination of fat-storing seeds. Planta (Berl.) 93, 269–288 (1970).
Heidrich, H.-G.: New aspects on the heterogeneity of beef liver catalase. Hoppe-Seylers Z. physiol. Chem. 349, 873–880 (1968).
Hock, B., Beevers, H.: Development and decline of the glyoxylate cycle enzymes in watermelon seedlings (Citrullus vulgaris Schrad.) Effects of dactinomycin and cycloheximide. Z. Pflanzenphysiol. 55, 405–414 (1966).
Hruban, Z., Rechcigl, M.: Microbodies and related particles. Int. Rev. Cytol., Suppl. I. New York: Academic Press 1969).
Hu, A. S. L., Bock, R. M., Halvorson, H. D.: Separation of labeled from unlabeled proteins by equilibrium density gradient sedimentation. Analyt. Biochem. 4, 489–504 (1962).
Kagawa, T., Beevers, H.: Glyoxysomes and peroxisomes in watermelon seedlings. Plant Physiol. 46, S-38 (1970).
Legg, P. G., Wood, R. L.: Effect of catalase inhibitors on the ultrastructure and peroxisome activity of proliferating microbodies. Histochemie 22, 262–276 (1970).
Longo, C. P.: Evidence for de novo synthesis of isocitratase and malate synthetase in germinating peanut cotyledons. Plant Physiol. 43, 660–664 (1968).
McGregor, D. J., Beevers, H.: Development of enzymes in watermelon seedlings. Plant Physiol. 44, S-33 (1969).
Poucke, M. van, Cerff, R., Barthe, F., Mohr, H.: Simultaneous induction of glycolate oxidase and glyoxylate reductase in white mustard seedlings by phytochrome. Naturwissenschaften 57, 132–133 (1970).
Radin, J. W., Breidenbach, R. W.: Development patterns of glyoxysomal and peroxisomal enzymes in cotyledons of safflower. Plant Physiol. 47, S-28 (1971).
Schmalfuss, K.: Die wirtschaftliche Bedeutung der Pflanzenfette und Fettpflanzen. Handbuch der Pflanzenphysiologie, Bd. VII, S. 354–375 (Ruhland, W., Hrsg.) Berlin-Göttingen-Heidelberg: Springer 1957.
Schnarrenberger, C., Oeser, A., Tolbert, N. E.: Development of microbodies in sunflower cotyledons and castor bean endosperm during germination. Plant Physiol. 48, 566–574 (1971).
Schnarrenberger, C., Oeser, A., Tolbert, N. E.: Isolation of protein bodies on sucrose gradients. Planta (Berl.) 104, 185–194 (1972).
Schopfer, P., Hock, B.: Nachweis der Phytochrom-induzierten de novo-Synthese von Phenylalaninammoniumlyase (PAL, E.C.4.3.1.5) in Keimlingen von Sinapis alba L. durch Dichtemarkierung mit Deuterium. Planta (Berl.) 96, 248–253 (1971).
Tolbert, N. E., Yamazaki, R. K., Oeser, A.: Localization and properties of hydroxypyruvate and glyoxylate reductases in spinach leaf particles. J. biol. Chem. 245, 5129–5136 (1970).
Trelease, R. N., Becker, W. M., Gruber, P. J., Newcomb, E. H.: Microbodies (glyoxysomes and peroxisomes) in cucumber cotyledons. Correlative biochemical and ultrastructural study in light- and dark-grown seedlings. Plant Physiol. 48, 461–475 (1971).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Gerhardt, B. Untersuchungen zur Funktionsänderung der Microbodies in den Keimblättern von Helianthus annuus L.. Planta 110, 15–28 (1973). https://doi.org/10.1007/BF00386919
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00386919