Abstract
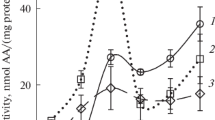
Glutathione transferase (GST) activity revealed in vacuoles of red beetroot (Beta vulgaris L.) cells was investigated in comparison with the GST activity of plastids and extracts of tissues. The level of GST activity determined by spectrophotometric method proved fairly high in water extracts and membrane fractions of isolated vacuoles and plastids, as well as in water extracts of tissues. In the objects studied, pH dependence of the GST activity slightly differed. Optimal pH for the vacuolar GST activity was in the range 7.0–7.5, for the GST of plastids and tissue extracts it was 7.5. The GSTs differed in specificity to the substrates fluorodifen and ethacrynic acid. The activity of the vacuolar and tissue extract GSTs with fluorodifen was significantly higher than that of the GST from plastids. Ethacrynic acid, often used as a competitive inhibitor of GST, almost completely inhibited the GST activity assayed with 1-chloro-2,4-dinitrobenzene as a main substrate. However, ethacrynic acid was a substrate only for the GSTs of vacuoles and tissue extract, but not for the GST of plastids. Using zymography allowing estimation of the GST activity in a gel after electrophoresis of proteins, several zones of enzymatic activity were revealed in all objects that may correspond to different isozymes. It was found that the composition of the vacuolar GST isoforms and their substrate specificity may differ from the GSTs of other cellular structures. It is assumed that vacuole, having quite high activity of GST, should make a significant contribution to intracellular detoxification processes.
Similar content being viewed by others
Abbreviations
- CDNB:
-
1-chloro-2,4-dinitrobenzene
- EA:
-
ethacrynic acid
- FD:
-
fluorodifen
- GSH:
-
reduced form of glutathione
- GST:
-
glutathione-S-transferase
- MDH:
-
malate dehydrogenase
References
Riechers D.E., Zhang Q., Xu F., Vaughn K.C. 2003. Tissue-specific expression and localization of safenerinduced glutathione S-transferase proteins in Triticum tauschii. Planta. 217 (5), 831–840.
Mohsenzadeh S., Esmaeili M., Moosavi F., Shahrtash M., Saffari B., Mohabatkar H. 2011. Plant glutathione S-transferase classification, structure and evolution. Afr. J. Biotechnol. 10 (42), 8160–8165.
Öztetik E.A. 2008. Tale of plant glutathione S-transferases: Since 1970. Bot. Rev. 74 (3), 419–437.
Droog F., Hooykaas P., Van der Zaal B.J. 1995. 2,4- Dichlorophenoxyacetic acid and related chlorinated compounds inhibit two auxin-regulated type-III tobacco glutathione S-transferases. Plant Physiol. 107 (4), 1139–1146.
Edwards R., Dixon D.P., Walbot V. 2000. Plant glutathione S-transferase: Enzymes with multiple functions in sickness and in health. Trends Plant Sci. 5 (5), 193–198.
Edwards R., Dixon D.P. 2005. Plant glutathione transferases. Meth. Enzymol. 401, 169–186.
Dixon D.P., Davis B.G., Edwards R. 2002. Functional divergence in the glutathione transferase superfamily in plants. Identification of two classes with putative functions in redox homeostasis in A. thaliana. J. Biol. Chem. 277 (34), 30859–30869.
Deponte M. 2013. Glutathione catalysis and the reaction mechanisms of glutathione-dependent enzymes. Biochim. Biophys. Acta. 1830 (5), 3217–3266.
Meyer A.J., Rausch T. 2008). Biosynthesis, compartmentation and cellular functions of glutathione in plant cells. In: Sulfur metabolism in phototrophic organisms. Eds. Hell R., Dahl C., Knaff D., Leustek T. Dordrecht: Springer Verlag, p. 165–188.
Flury T., Wagner E., Kreuz K. 1996. An inducible glutathione S-transferase in soybean hypocotylsis localized in the apoplast. Plant Physiol. 112 (3), 1185–1190.
Takahashi Y., Hasezawa S., Kusaba M., Nagata T. 1995. Expression of the auxin-regulated parA gene in transgenic tobacco and nuclear localization of its gene product. Planta. 196 (1), 111–117.
Carter C., Pan S., Zouhar J., Avila E.L., Girke T., Raikhel N.V. 2004. The vegetative vacuole proteome of Arabidopsis thaliana reveals predicted and unexpected proteins. Plant Cell. 16 (12), 3285–3303.
Dixon D., Hawkins T., Hussey J. Edwards R. 2009. Enzyme activities and subcellular localization of members of the Arabidopsis glutathione transferase superfamily. J. Ex. Bot. 60 (4), 1207–1218.
Leigh R.A., Branton D. 1976. Isolation of vacuoles from root storage tissue of Beta vulgaris L. Plant Physiol. 58 (5), 656–662.
Kuzevanov V.Ya, Katkov B.B., Saliaev R.K. 1985). General principles of isolation of vacuoles and vacuolar membranes. In: Struktura i funktsii biologicheskikh membran rastenii (Structure and function of plant biological membranes). Eds. Saliaev R.K., Voinikov V.K. Novosibirsk: Nauka, p. 93–107.
Asada K., Badger M.R. 1984. Photoreduction of 18O2 and H2 18O2 with concomitant evolution of 16O2 in intact spinach chloroplasts: Evidence for scavenging of hydrogen peroxide by peroxidase. Plant Cell Physiol. 25 (7), 1169–1179.
Edwards G.E., Nakamoto H., Bunell J.N., Hatch M.D. 1985. Pyruvate, Pi dikinase and NADP-malate dehydrogenase in C4 photosynthesis: Properties and mechanism of light/dark regulation. Annu. Rev. Plant Physiol. 36, 255–286.
Yudina R.S. 2012. Malate dehydrogenase in plants: Its genetics, structure, localization and use as a marker. Advances in Bioscience and Biotechnology. 3 (4), 370–377.
Levites E.V., 1986. Genetika izofermentov rastenii (Genetics of plant isoenzymes). Novosibirsk: Nauka, Siberian Branch.
Habig W.H., Pabst M.J., Jakoby W.B. 1974. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 249 (22), 7130–7139.
Kilili K.G., Atanassova N., Vardanyan A. Clatot N., Al-Sabarna K., Kanellopoulos P.N., Makris A.M., Kampranis S.C. 2004. Differential roles of tau class glutathione S-transferases in oxidative stress. J. Biol. Chem. 279 (23), 24540–24551.
Pascal S., Scalla R. 1999. Purification and characterization of a safener-induced glutathione S-transferase from wheat (Triticum aestivum). Physiol. Plant. 106 (1), 17–27.
Bradford M. 1976. A rapid and sensitive method for the quantitation of protein utilising the principal of protein- dye binding. Anal. Biochem. 72, 248–254.
Gaal O., Medgyesi G.A., Vereczke, L. 1980. Electrophoresis in the Separation of Biological Macromolecules. New York: John Wiley and Sons.
Gupta S., Rathaur S. 2005. Filarial glutathione S-transferase: Its induction by xenobiotics and potential as drug target. Acta Biochim. 52 (2), 493–500.
Marrs K.A. 1996. The functions and regulation of glutathione S-transferases in plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 47, 127–158.
Zybailov B., Rutschow H., Friso G., Rudella A., Emanuelsson O., Sun Q., Van Wijk K.J. 2008. Sorting signals, N-terminal modifications and abundance of the chloroplast proteome. PLoS One. 3 (4). doi 10.1371journalpone.0001994
Edwards R., Cole D.J. 1996. Glutathione transferases in wheat (Triticum) species with activity toward fenoxaprop-ethyl and other herbicides. Pestic. Biochem. Physiol. 54 (2), 96–104.
Cummins I., Cole D.J., Edwards R. 1997. Purification of multiple glutathione transferases involved in herbicide detoxification from wheat (Triticum aestivum L.) treated with the safener fenchlorazole-ethyl. Pestic. Biochem. Physiol. 59 (1), 35–49.
Zettl R., Schell J., Palme K. 1994. Photoaffinity labeling of Arabidopsis thaliana plasma membrane vesicles by 5-azido-[7-3H]indole-3-acetic acid: Identification of a glutathione S-transferase (photoafinity labeling/auxinbinding protein). Plant Biology. 91 (2), 689–693.
DeRidder B., Dixon D., Beussman D.J. Edwards R., Goldsbrough P.B. 2002. Induction of glutathione S-transferases in Arabidopsis by herbicide safeners. Plant Physiol. 130 (3), 1497–1505.
Mozer T.J., Tiemeier D.C., Jaworski E.G. 1983. Purification and characterization of corn glutathione S-transferase. Biochemistry. 22 (5), 1068–1072.
Zeng Q.-Y., Lu H., Wang X.-R. 2005. Molecular characterization of a glutathione transferase from Pinus tabulaeformis (Pinaceae). Biochimie. 87 (5), 445–455.
Dixon D.P., Cole D.J., Edwards R. 1998. Purification, regulation and cloning of a glutathione transferase (GST) from maize resembling the auxin-inducible type-III GSTs. Plant Mol. Biol. 36 (1), 75–87.
Cottingham C.K., Hatzios K.K., Meredith S. 1998. Influence of chemical treatments on glutathione S-transferases of maize with activity towards metolachlor and cinnamic acid. Z. Naturforsch. 53 (11–12), 973–979.
Katkov B.B., Korzun A.M., Saliaev R.K. 1990. Preservation of native features of beetroot vacuoles, isolated in solutions of KCL and sorbitol. Fisiologiya rasteniy (Rus). 37 (2), 362–371.
Phillips M.F., Mantle T.J. 1991. The initial-rate kinetics of mouse glutathione S-transferase YfYf. Evidence for an allosteric site for ethacrynic acid. Biochem. J. 275 (3), 703–709.
Nóvoa-Valiñas M.C. Pérez-López M., Melgar M.J. 2002. Comparative study of the purification and characterization of the cytosolic glutathione S-transferases from two salmonid species: Atlantic salmon (Salmo salar) and brown trout (Salmo trutta). Comp. Biochem. Physiol. C. Toxicol. Pharmacol. 131 (2), 207–213.
Cummins I., Cole D.J., Edwards R. 1999. A role for glutathione S-transferase functioning as glutathione peroxidases in resistance to multiple herbicides in black-grass. Plant J. 18 (3), 285–292.
Dixon D., Cummins L., Cole D.J., Edwards R. 1998. Glutathione-mediated detoxification system in plants. Curr. Opin. Plant Biol. 1 (3), 258–266.
Irzyk C.P., Fuerst E. 1993. Purification and characterization of a glutathione S-transferase from benoxacortreated maize (Zea mays). Plant Physiol. 102 (3), 803–810.
Gronwald J.W., Plaisance K.L. 1998. Isolation and characterization of glutathione S-transferase isozymes from sorghum. Plant Physiol. 117 (3), 877–892.
Deng F., Nagao A., Shim I.S., Usui K. 1996. Induction of glutathione S-transferase isozymes in rice shoots treated with a combination of pretilachlor and fenclorim. J. Weed Sci. Tech. 42 (3), 277–283.
Nimaeva O.D., Pradedova E.V., Saliaev R.K. 2014. Activity and isoenzyme composition of vacuolar peroxidase from the cells of beetroot at various stages of ontogenesis and after changes in storage conditions. Fisiologiya rasteniy (Rus.). 61 (3), 350–358.
Frova C. 2006. Glutathione transferases in the genomics era: New insights and perspectives. Biomol. Eng. 23 (4), 149–169.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © E.V. Pradedova, O.D. Nimaeva, I.S. Truchan, R.K. Salyaev, 2016, published in Biologicheskie Membrany, 2016, Vol. 33, No. 2, pp. 140–149.
Rights and permissions
About this article
Cite this article
Pradedova, E.V., Nimaeva, O.D., Truchan, I.S. et al. Glutathione transferase activity of vacuoles, plastids, and tissue extracts of red beetroot. Biochem. Moscow Suppl. Ser. A 10, 223–232 (2016). https://doi.org/10.1134/S1990747816020082
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S1990747816020082