Abstract
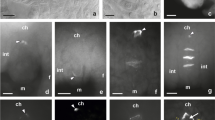
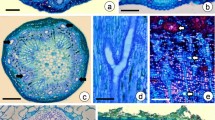
Abscisic acid (ABA) treatment of secondary protonema of Physcomitrium pyriforme Brid in the presence of sucrose does not prevent cell division but results in shorter cells with vesicular cytoplasm and an accumulation of lipid. When transferred to sucrose medium without ABA and with low irradiance isodiametric intercalary cells are cut off which give rise to apogamous sporophytes either directly or after the formation of a small amount of callus. The organization of the cells leading up to the apogamous sporophyte is described. The cells initiating the sporophyte develop dense cytoplasm and the walls become labyrinthine and callosed, but they do not form any recognizable placenta. It is proposed that labyrinthine walls are a consequence of a perturbation of cell wall metabolism as growth changes from gametophytic to sporophytic. The use of the term “transfer cell” for this kind of cell is questioned and the need for a causal approach to the investigation of labyrinthine walls is stressed.
Similar content being viewed by others
References
Barthelmess, A. (1941) Mutationsversuche mit einem Laubmoos Physcomitrium pyriforme. II. Morphologische und physiologische Analyse der univalenten und bivalenten Protonemen einiger Mutanten. Z. Indukt. Abstamm. Vererbungsl. 79, 153–170
Bauer, I. (1967) Determination von Gametophyt und Sporophyt. In: Encyclopedia of plant physiology XVIII, pp. 235–256, Ruhland, W. ed. Springer, Berlin Heidelberg New York
Bell, P.R. (1961) Failure of nucleotides to diffuse freely into the embryo of Pteridium aquilinum. Nature (London) 191, 91–92
Bertlanffy, S. (1963) Acridine orange fluorescence in cell physiology cytochemistry and medicine. Protoplasma 57, 51–83
Bopp, M. (1976) External and internal regulation of the differentiation of the moss protonema. J. Hattori. Bot. Lab. 41, 167–177
Browning, A.J., Gunning, B.E.S. (1979) Structure and function of transfer cells in the sporophyte haustorium of Funaria hygrometrica Hedw. II. Kinetics of uptake of labelled sugars. J. Exp. Bot. 30, 1247–1264
Colquhoun, A.J., Hillman, J.R., Crewe, C., Bowes, B.G. (1975) An ultrastructural study of the effects of abscisic acid on senescence of leaves of radish (Raphanus sativus L.). Protoplasma 84, 205–221
Dodds, J.H., Phillips, R. (1977) DNA and histone content of immature tracheary elements from cultured artichoke explants. Planta 135, 213–216
Erichsen, J., Knoop, B., Bopp, M. (1977) On the action mechanism of cytokinins in mosses: caulonema specific proteins. Planta 135, 161–168
Feder, N., O'Brien, T.P. (1968) Plant microtechnique: some principles and new methods. Am. J. Bot. 55, 123–142
Fischer, A. (1884) Untersuchungen über das Siebröhren — System der Cucurbitaceen. Borntraeger, Berlin
Gunning, B.E.S., Pate, J.S., Briarty, L.G. (1968) Specialized “transfer cells” in minor veins of leaves and their possible significance in phloem translocation. J. Cell Biol. 37, C7
Jensen, W.A. (1962) Botanical histochemistry. Freeman, San Francisco London
Knoop, B. (1978) Multiple DNA contents in the haploid protonema of the moss Funaria hygrometrica Sibth. Protoplasma 94, 307–314
Lal, M., Bell, P.R. (1977) Aspects of the differentiation of the egg of the moss Physcomitrium coorgense Broth. Ann. Bot. 41, 127–131
Lehmann, H., Schulz, D. (1969) Elektronemikroskopische Untersuchungen von Differenzierungsvorgängen bei Moosen. II. Die Zellplatten und Zellwandbildung. Planta 85, 313–325
Maier, K., Maier, U. (1972) Localization of Beta-glycerophosphatase and Mg2+-activated adenosine triphosphatase in a moss haustorium. Protoplasma 75, 91–112
Menon, M.K.C., Lal, M. (1972) Influence of sucrose on the differentiation of cells with zygote-like potentialities in a moss. Naturwissenschaften 59, 514
Menon, M.K.C., Lal, M. (1974) Morphogenetic role of kinetin and abscisic acid in the moss Physcomitrium. Planta 115, 319–328
Menon, M.K.C., Lal, M. (1977) Regulation of a subsexual life cycle in a moss. Evidence for the occurrence of a factor for apogamy in Physcomitrium. Ann. Bot. 41, 1179–1189
Milborrow, B.V. (1974) The chemistry and physiology of abscisic acid. Annu. Rev. Plant Physiol. 25, 259–307
Mittelheuser, C.J., Van Steveninck, R.F.M. (1971a) The ultrastructure of wheat leaves. I. Changes due to natural senescence and the effects of kinetin and ABA on detached leaves incubated in the dark. Protoplasma 73, 239–252
Mittelheuser, C.J., Van Steveninck, R.F.M. (1971b) The ultrastructure of wheat leaves. II. The effects of kinetin and ABA on detached leaves incubated in the light. Protoplasma 73, 253–262
Mlodzianowsky, F., Szweykowska, A. (1971) Fine structure of kinetin-treated protonema and kinetin-induced gametophoric buds in Funaria hygrometrica. Acta Soc. Bot. Pol. 40, 549–555
Moore, G.T. (1903) Methods for growing pure cultures of algae. J. Appl. Microsc. 6, 2309–2314
Reynolds, E.S. (1963) The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J. Cell Biol. 17, 208–212
Ripetsky, R.T., Demkiv, O.T., Lavrinkh, T.V. (1972) Cytofluorimetry of cellular nuclei in the process of differentiation of moss protonema. Ukr. Bot. J. 29, 315–320
Schmiedel, G., Schnepf, E. (1980) Polarity growth of caulonema tip cells of the moss Funaria hygrometrica. Planta 147, 405–413
Spurr, A.R. (1969) A low viscosity epoxy resin embedding medium for electron microscopy. J. Ultrastruct. Res. 26, 31–43
Thiéry, J.P. (1967) Mise en évidence des polysaccharides sur coupes fines en microscopie electronique. J. Microsc. 6, 987–1018
Thorpe, T.A., Meier, D.D. (1972) Starch metabolism, respiration and shoot formation in tobacco callus cultures. Physiol. Plant. 27, 365–369
Valanne, N. (1971) Environmentally induced formation of plasmatic filaments in the protonema of Ceratodon purpureus. Protoplasma 73, 97–105
Valanne, N. (1974) Autoradiographic investigation of incorporation of tritiated sucrose and 1-methionine in the protonema of Ceratodon purpureus. Protoplasma 82, 237–247
Woodcock, C.L.F., Bell, P.R. (1967) A method for mounting 4 μm resin sections routinely for ultrathin sectioning. J. R. Microsc. Soc. 87, 485–487
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Menon, M.K., Bell, P.R. Ultrastructural and cytochemical aspects of induced apogamy following abscisic acid pre-treatment of secondary moss protonema. Planta 151, 427–433 (1981). https://doi.org/10.1007/BF00386535
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00386535