Abstract
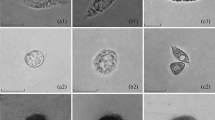
To study the usefulness of haemolymph for shrimp health assessment characteristics of both the cellular and humoral composition of growing juvenile Penaeus monodon (n = 16) were determined. Haemolymph was obtained by a puncture of the ventral part of the haemocoel. Using routine staining methods five different cell types could be distinguished by light microscopy. Electron microscopy studies revealed granulocytes, semigranulocytes and hyalinocytes. Mean total haemocyte count was 50.9 × 106 cells/ml whereas mean haemolymph plasma protein content was 79.9 g/l consisting of one major and one or two minor fractions.
It was concluded that haemolymph characterisation can be a useful tool for health estimation of P. monodon, but standardisation of the techniques is needed.[/p]
Similar content being viewed by others
References
Adams A (1991) Response of penaeid shrimp to exposure to Vibrio species. Fish Shellfish Immunol 1:59–70
Anonymous (1994) Aquaculture production 1985–1992. FAO Fisheries Circular No. 815 Rev. 6. Rome, Italy, 213 pp
Bachère E, Mialhe E, Noël D et al. (1995) Knowledge and research prospects in marine mollusc and crustacean immunology. Aquaculture 132:17–32
Balazs GH, Olbrich SE, Tumbleson ME (1974) Serum constituents of the Malaysian prawn (Macrobrachium rosenbergii) and pink shrimp (Penaeus marginatus). Acquaculture 3:147–157
Bang FB (1971) Transmissable disease, probably viral in origin, affecting the amoebocyte of European shore crab Carcinus maenas. Infect Immunol 3:617–623
Baticados MCL, Lavilla-Pitogo CR, Cruz-Lacierda, ER et al. (1990) Studies on the chemical control of luminous bacteria Vibrio harveyi and V. splendidus isolated from diseased Penaeus monodon larvae and rearing water. Dis Aquat Org 9:133–139
Bodhipaksha N, Weeks-Perkins BA (1994) The effects of methyl parathion on phagocytosis and respiratory burst activity of tiger shrimp (Penaeus monodon) phagocytes. In: Stolen JS, Fletcher TC (eds) Modulaters of fish immune responses. Vol 1, SOS Publications, Fair Haven, USA, pp 11–22
Borchard B (1978) Studies on the rainbow trout (Salmo gairdneri). I. Correlation between gonadal development and serum protein patterns. Arch Int Physiol Biochem Biophys 18:1027–1034
Brock JA (1992) Current diagnostic methods for agents and diseases of farmed marine shrimp. In: Fulks W, Main KL (eds) Diseases in cultured penaeid shrimp in Asia and the United States. Proceedings of a workshop in Honolulu, Hawaii, 27–30 April, 1992. The Oceanic Institute, Honolulu, Hawaii, pp 209–233
Bursey CR, Lane CE (1971) Ionic and protein concentrations changes during the molt cycle of Penaeus duodarum. Comp Biochem Physiol 40A:155–162
Chan SM, Rankin SM, Keeley LL (1988) Characterization of the molt stages in Penaeus vannamei: setogenesis and hemolymph levels of total protein, ecdysteroids, and glucose. Biol Bull 175:185–192
Chang CF, Jeng SR (1995) Isolation and characterization of the female-specific protein (vitellogenin) in mature female hemolymph of the prawn Penaeus chinsensis. Comp Biochem Physiol 112B:258–263
Chang CF, Lee FY, Huang YS et al. (1994) Purification and characterization of the female-specific protein (vitellogenin) in mature female hemolymph of the prawn, Penaeus monodon. Invert Reprod Dev 25:185–192
Chantanachookin C, Boonyaratpalin S, Kasornchandra J et al. (1993) Histology and ultrastructure reveal a new granulosis-like virus in Penaeus monodon affected by yellow-head disease. Dis Aquat Org 17:145–57
Chen JC, Cheng SY (1993) Studies on hemocyanin and haemolymph protein levels of Penaeus japonicus based on sex, size and moulting cycle. Comp Biochem Physiol 106B:293–296
Chen JC, Liu PC, Lin YT (1989) Culture of Penaeus monodon in an intensified system in Taiwan. Aquaculture 77:319–328
Chen JC, Chen CT, Cheng SY (1994a) Nitrogen excretion and changes of hemocyanin, protein and free amino acid levels in the hemolymph of Penaeus monodon exposed to different concentrations of ambient ammonia-N at different salinity levels. Mar Ecol Prog Serv 110:85–94
Chen JC, Cheng SY, Chen CT (1994b) Changes of hemocyanin, protein and free amino acid levels in the haemolymph of Penaeus japonicus exposed to ambient ammonia. Comp Biochem Physiol 109A:339–347
Chen SN (1995) Current status of shrimp aquaculture in Taiwan. In: Browdy CL, Hopkins JS (eds) Swimming through troubled water. Proceedings of the special session on shrimp farming, Aquaculture '95, The World Aquaculture Society, Louisiana, USA, pp 29–34
Chisholm JRS, Smith VJ (1995) Comparison of antibacterial activity in the hemocytes of different crustacean species. Comp Biochem Physiol 110A:39–45
Cornick JW, Stewart JE (1973) Partial characterization of a natural agglutinin in the hemolymph of the lobster Homarus americanus. J Invert Pathol 21:255–262
Dall W (1974) Indices of nutritional state in the western rock lobster, Panulirus langipes (Milne Edwards). I. Blood and tissue constituents and water content. J Exp Mar Biol Ecol 16:167–180
Ferraris RP, Parado-Estepa FD, Ladja JM et al. (1986) Effect of salinity on the osmotic, chloride, total protein and calcium concentrations in the hemolymph of the prawn Penaeus monodon (Fabricius). Comp Biochem Physiol 83A:701–708
Ferrero EA, Graziosi G, Mazari R et al. (1983) Rapid communication. Protein pattern variability of hemolyumph of Mantis shrimp, Squilla mantis L. (Crustacea, Stomatopoda). J Exp Zool 225:341–345
Ford SE (1986) Effect of repeated hemolymph sampling on growth, mortality, hemolymph protein and parasitism of oysters, Crassostrea virginica. Comp biochem Physiol 85A:465–470
Friebel B, Renwrantz L (1995) Application of density gradient centrifugation for separation of eosinophilic and basophilic hemocytes from Mytilus edulis and characterization of both cell groups. Comp Biochem Physiol 112A:81–90
Hose JE, Martin GG (1989) Defense functions of granulocytes in the Ridgeback prawn Sicyonia ingentis. J Invert Pathol 53:335–346
Hose JE, Martin GG, Nguyen VA et al. (1987) Cytochemical features of shrimp hemocytes. Biol Bull 173:178–187
Itami T, Takahashi Y, Nakamura Y (1989) Efficacy of vaccination against Vibriosis in cultured Kuruma prawns Penaeus japonicus. J Aquat Anim Health 1:238–242
Koumans-Van Diepen JCE, Harmsen EGM, Rombout JHWM (1994) Immunocytochemical analysis of mitogen responses of carp (Cyprinus carpio L.) peripheral blood leucocytes. Vet Immunol Immunopathol 42:209–219
Martin GG, Graves BL (1985) Fine structure and classification of shrimp hemocyts. J Morphol 185:339–348
McKay D, Jenkin CR (1970) Immunity in the invertebrates. The role of serum factors in phagocytosis of erythrocytes by haemocytes of the freshwater crayfish (Parachaeraps bicarinatus). Aust J Exp Biol Med Sci 48:139–150
Meyer F (1991) Aquaculture disease and health management. J Anim Sci 69:4201–4208
Mialhe E, Bachère E, Boulo V et al. (1995) Strategy for research and international cooperation in marine invertebrate pathology, immunology and genetics. Aquaculture 132:33–41
Miranpuri GS, Bidochka MJ, Khachatourians GG (1991) Morphology of cytochemistry of hemocytes and analysis of hemolymph from Melanoplus sanguinipes (Orthoptera: Acrhididae). J Econ Entomol 84:371–378
Oliver LM, Fisher WS (1995) Comparative form and function of oyster Crassostrea virginica hemocytes from Chesapeake Bay (Virginia) and Apalachicola Bay (Florida). Dis Aquat Org 22:217–225
Paterson WD, Keith IR (1992) Disease and defense mechanisms of the American lobster, Homarus americanus. In: Shariff M, Subasinghe RP, Arthur JR (eds) Diseases in Asian aquculture. I. Proceedings of the first symposium on diseases in Asian aquaculture, 26–29 November, 1990, Bali, Indonesia, pp 81–88
Pelc R (1986) The haemocytes and their classification in the larvae and pupae of Mamestra brassicae (L.) 1758 (Lepidoptera; Noctuidae). Can J Zool 64:2503–2508
Plumb JA (1992) Disease control in aquaculture. In: Shariff M, Subassinghe RP, Arthur JR (eds) Diseases in Asian aquaculture. I. Proceedings of the first syumposium on diseases in Asian aquaculture, 26–29 November, 1990. Bali, Indonesia, pp 3–19
Rodriguez J, Boulo V, Mialhe E et al. (1995) Characterisation of shrimp haemocytes and plasma components by monoclonal antibodies. J Cell Science 108:1043–1050
Romeis B (1968) Mikroscopische technik. Oldenbourg Verlag, München, 757 pp
Rosenberry B (1995) World shrimp farming 1995. Shrimp News International, San Diego, USA, 68 pp
Schippers C, Ulloa-Rojas JB, Booms GHR et al. (1994) A dietary effect on some cellular and humoral blood parameters of rainbow trout, Oncorynchus mykiss (Walbaum) Aquac Fish Man 25:649–657
Shiu-Nan C (1992) Coping with diseases in shrimp farming. In: Saram H de, Singh T (eds) Shrimp '92, Hong Kong. Proceedings of the 3rd global conference on the shrimp industry Hong Kong, 14–16 September, 1992. Infofish, Malaysia, pp 113–117
Smith VJ, Chisholm JRS (1992) Non-cellular immunity in crustaceans. Fish Shellfish Immunol 2:1–31
Spicer JI, Taylor AC (1987) Ionic regulation and salinity related changes in haemolymph protein in the semi-terrestrial beachflea Orchestia gammarellus (Pallas) (Crustacea: Amphipoda). Comp biochem Physiol 88A:243–246
Steward JE, Cornick JW, Dingle JR (1967) An electronic method for counting lobster (Homarus americanus Milne Edwards) hemocytes and the influence of diet on hemocyte numbers and hemolymph proteins. Can J Zool 45:291–304
Sung HH, Kou GH, Song YL (1994) Vibrosis resistance induced by glucan treatment in Tiger Shrimp (Penaeus monodon). Fish Pathol 29:11–17
Söderhäll K, Cerenius L (1992) Crustacean immunity. Ann Rev Fish Dis. Pergamon Press Ltd, USA, pp 3–23
Söderhäll S, Johansson MW, Smith VJ (1988) Internal defense mechanisms. In: Holdich DM, Lowery RS (eds) Freshwater crayfish: biology, management and exploitation. Croom Held, London, pp 213–235
Triebskorn R, Köhler HR, Zhan T et al. (1991) Invertebrate cells as targets for hazardous substances. Zeitschr Agnew Zool 78:277–287
Tsing A, Arcier JM, Brehélin M (1989) Hemocytes of Penaeid and Palaemonid shrimps: morphology, cytochemistry, and hemograms. J Invert Pathol 53:64–77
Ullrich B, Vollmer M, Stöcker W, et al. (1992) Hemolymph protein patterns and coprophagous behaviour in Oniscus asellus L. (Crustacea, Isopoda). Invert Reprod Dev 21:193–200
Vargas-Albores F, Guzman A, Ochoa JL (1992) Size-dependent haemagglutinating activity in the haemolymph from sub-adult blue shrimp (Penaeus stylirostris Stimpson). Comp Biochem Physiol 103A:487–491
Yeager JF, Tauber OE (1935) On the hemolymph cell counts of some marine invertebrates. Biol Bull 69:66–70
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
van de Braak, C.B.T., Faber, R. & Boon, J.H. Cellular and humoral characteristics of Penaeus monodon (Fabricius, 1798) haemolymph. Comparative Haematology International 6, 194–203 (1996). https://doi.org/10.1007/BF00378110
Issue Date:
DOI: https://doi.org/10.1007/BF00378110