Abstract
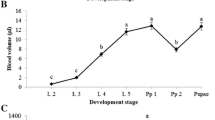
The aim of this work was to conduct a morphological analysis of hemocytes of the Australian red claw crayfish (Cherax quadricarinatus). Hemocytes were studied in the native and anticoagulant-treated hemolymph and stained with May–Grünwald and Romanowsky stains. Hemolymph was collected with a syringe from the ventral sinus. Microscopy was carried out using 40× or 100× objective lenses. Hemocyte count and the percentage of each of their types were determined in the Goryaev chamber. In the hemolymph of C. quadricarinatus, among the already known types of hemocytes (agranulocytes or hyalinocytes, semigranulocytes, and granulocytes), we found the cells morphologically different from the previously described, transparent cells. Hyalinocytes are oval or fusiform cells, about 26.6 µm long and 9.2 µm wide. As a rule, they lack granules, but sometimes a small number of tiny granules, sized less than 0.5 µm, can be found. Cells of this type are able to outlive other cells on a slide after isolation. Semigranulocytes are also oval or fusiform cells, 26.7 µm long and 9.3 µm wide, with a moderate number of small-sized granules (<0.5 µm) and sparse medium-sized granules ranging from 0.77 to 1.69 µm. Granulocytes are oval-shaped cells, the largest of all types, having a length and width of 28.7 and 11.1 µm, respectively. They contain large (0.8–2.48 µm) numerous granules and show a high refraction, due to which this cell type is well recognizable under a microscope. These cells have a lowest nuclear–cytoplasmic ratio compared to the former two types. Transparent cells are specifically characterized by abundant well-developed pseudopodia. After cell rounding, its diameter averages 10.7 ± 1.11 µm. This cell type begins to show up 10 min after their isolation in the anticoagulant-untreated hemolymph. Hyalinocytes are the dominant type of cells in the hemolymph, their proportion is 48.3 ± 11.4%, while semigranulocytes and granulocytes account for 26.3 ± 7.8 and 25.2 ± 6.9%, respectively. The total number of hemocytes varies in a wide range from 820 to 5510 cell/µL, with the average number of cells being 2707 ± 1096 cell/µL. The proportion of transparent cells averages 18.2 ± 3.8%.








Similar content being viewed by others
REFERENCES
Johansson MW, Keyser P, Sritunyalucksana K, Söderhäll K (2000) Crustacean haemocytes and haematopoiesis. Aquaculture 191(1–3): 45–52. https://doi.org/10.1016/s0044-8486(00)00418-x
Cerenius L, Jiravanichpaisal P, Liu H, Söderhäll I (2010) Crustacean Immunity. In: Söderhäll K (ed) Invertebrate Immunity. Advanc Experiment Med Biol. Springer, Boston; MA, 708. https://doi.org/10.1007/978-1-4419-8059-5_13
Alexandrova EH, Kovatcheva NP (2010) In life determination of the physiological status of decapod crustaceans (Crustacea: Decapoda) by hematological characteristics. Prog Physiol Sci 41(2): 51–67. (In Russ).
Kovatcheva NP, Aleksandrova EN (2010) Hematological parameters as an indicators of physiological status of the decapods: red king crab Paralithodes camttshaticus and freshwater crayfish genus Astacus and Pontastacus. VNIRO Publishing, Moscow. (In Russ).
Adzhiev DD, Pronina GI, Ivanov AA, Koryagina NYu (2018) Functional indicators of poikilothermic aquatic species from natural and artificial water biocenoses. Sel’skokhoz biol 53(2): 337–347. https://doi.org/10.15389/agrobiology.2018.2.337rus
Ivanov AA, Pronina GI, Koryagina NYu, Revyakin AO (2013) Internal environment homeostasis of hydrobionts: specific peculiarities of cold-blooded animals. Izvest Timiryazev Agricult Acad 3: 75–88 (In Russ).
Pronina GI, Koryagina NYu (2014) Comprehensive intravital physiological assessment of crayfishes in aquaculture. Theor Appl Probl Agro-industrial Complex 4: 46–48. (In Russ).
Roulston C, Smith VJ (2011) Isolation and in vitro characterisation of prohaemocytes from the spider crab, Hyas araneus (L.). Devel Comparat Immunol 35(5): 537–544. https://doi.org/10.1016/j.dci.2010.12.012
Mix MC, Sparks AK (1980) Hemocyte classification and differential counts in the dungeness crab, Cancer magister. J Invertebr Pathol 35(2): 134–143. https://doi.org/10.1016/0022-2011(80)90176-7
Sternshein DJ, Burton PR (1980) Light and electron microscopic studies of crayfish hemocytes. J Morphol 165(1): 67–83. https://doi.org/10.1002/jmor.1051650107
Martin GG, Graves BL (1985) Fine structure and classification of shrimp hemocytes. J Morphol 185: 339–348. https://doi.org/10.1002/jmor.1051850306
Tsing A, Arcier J-M, Brehélin M (1989) Hemocytes of Penaeid and Palaemonid shrimps: Morphology, cytochemistry, and hemograms. J Invertebr Pathol 53(1): 64–77. https://doi.org/10.1016/0022-2011(89)90075-x
Hose JE, Martin GG, Gerard AS (1990) A decapod hemocyte classification scheme integrating morphology, Cytochemistry, and Function. Biol Bull 178(1): 33–45. https://doi.org/10.2307/1541535
Jussila J (1997) Physiological responses of Astacid and Parastacid Crayfishes (Crustacea: Decapoda) to conditions of intensive culture. doc. dissertation. Perth: University of Kuopio.
Hijran YY, Hasan HA (2002) Haemocyte Classification and Differential Counts in the Freshwater Crab, Potamon fluviatilis. Tukr J Vet Anim Sci 26: 403–406.
Zhang ZF, Shao M, Kang KH (2006) Classification of haematopoietic cells and haemocytes in Chinese prawn Fenneropenaeus chinensis. Fish and Shellfish Immunol 21: 159–169. https://doi.org/10.1016/j.fsi.2005.11.003
Giulianini PG, Bierti M, Lorenzon S, Battistella S, Ferrero EA (2007) Ultrastructural and functional characterization of circulating hemocytes from the freshwater crayfish Astacus leptodactylus: Cell types and their role after in vivo artificial non-self challenge. Micron 38(1): 49–57. https://doi.org/10.1016/j.micron.2006.03.019
Li C, Shields JD (2007) Primary culture of hemocytes from the Caribbean spiny lobster, Panulirus argus, and their susceptibility to Panulirus argus Virus 1 (PaV1). J Invertebr Pathol 94(1): 48–55. https://doi.org/10.1016/j.jip.2006.08.011
Taylor S, Landman MJ, Ling N (2009) Flow сytometric characterization of freshwater crayfish hemocytes for the examination of physiological status in wild and captive animals. Journal of Aquatic Animal Health 21(3): 195–203. https://doi.org/10.1577/h09-003.1
Ding ZZ, Du JJ, Ou JJ, Li WW, Wu TT, Xiu YY, Meng QQ, Ren QQ, Gu WW, Xue HH, Tang JJ, Wang WW (2012) Classification of circulating hemocytes from the red swamp crayfish Procambarus clarkii and their susceptibility to the novel pathogen Spiroplasma eriocheiris in vitro. Aquaculture 356: 371–380. https://doi.org/10.1016/j.aquaculture.2012.04.042
Du J, Zhu H, Ren Q, Liu P, Chen J, Xiu Y, Yao W, Meng Q, Gu W, Wang W (2012) Flow cytometry studies on the Macrobrachium rosenbergii hemocytes sub-populations and immune responses to novel pathogen spiroplasma MR-1008. Fish and Shellfish Immunol 33(4): 795–800. https://doi.org/10.1016/j.fsi.2012.07.006
Lv SS, Xu JJ, Zhao JJ, Yin NN, Binjie Lu B, Li SS, Chen YY, Xu HH (2014) Classification and phagocytosis of circulating haemocytes in Chinese mitten crab (Eriocheir sinensis) and the effect of extrinsic stimulation on circulating haemocytes in vivo. Fish and Shellfish Immunol 39(2): 415–422. https://doi.org/10.1016/j.fsi.2014.05.036
Battison A, Cawthorn R, Horney B (2003) Classification of Homarus americanus hemocytes and the use of differential hemocyte counts in lobsters infected with Aerococcus viridans var. homari (Gaffkemia). J Invertebr Pathol 84(3): 177–197. https://doi.org/10.1016/j.jip.2003.11.005
Lagutkina LYu, Ponomarev SV (2008) New object of aquaculture—australian redclaw crayfish (Cherax quadricarinatus). Vestnik AGTU 6: 220–223. (In Russ).
Lagutkina LYu, Kuzmina EG, Taranina AA, Ahmedzhanova AB, Yasinskij VS, Ponomarev RA (2020) Factual support of practices to increase the efficiency of cultivation of tropical freshwater species. Bulletin of the Astrakhan State Technical University. Series: Fisheries 2: 94–105. https://doi.org/10.24143/2073-5529-2020-2-94-105
Lagutkina LYu, Kuzmina EG, Taranina AA, Ahmedzhanova AB, Yasinskij VS, Ponomarev RA (2020) Factual support of practices to increase the efficiency of cultivation of tropical freshwater species. Bull Astrakhan State Techn Univers Series: Fisheries 2: 94–105. https://doi.org/10.24143/2073-5529-2020-2-94-105
Li F, Zheng Z, Li H, Fu R, Xu L, Yang F (2021) Crayfish hemocytes develop along the granular cell lineage. Sci Rep 11(1): 13099. https://doi.org/10.1038/s41598-021-92473-9
Duan H, Jin S, Zhang Y, Li F, Xiang J (2014) Granulocytes of the red claw crayfish Cherax quadricarinatus can endocytose beads, E. coli and WSSV, but in different ways. Develop and Compar Immunol 46(2): 186–193. https://doi.org/10.1016/j.dci.2014.04.006
Li F, Chang X, Xu L, Yang F (2018) Different roles of crayfish hemocytes in the uptake of foreign particles. Fish and Shellfish Immunol 77: 112–119. https://doi.org/10.1016/j.fsi.2018.03.029
Li F, Xu L, Hui X, Huang W, Yang F (2019) Directed differentiation of granular cells from crayfish hematopoietic tissue cells. Fish and Shellfish Immunol 88: 28–35. https://doi.org/10.1016/j.fsi.2019.02.054
Zhu K, Yang F, Li F (2022) Molecular markers for hemocyte subpopulations in crayfish Cherax quadricarinatus. Devel and Comparat Immunol 132: 104407. https://doi.org/10.1016/j.dci.2022.104407
Mauro M, Arizza V, Arculeo M, Attanzio A, Pinto P, Chirco P, Badalamenti G, Tesoriere L, Vazzana M (2022) Haemolymphatic Parameters in Two Aquaculture Crustacean Species Cherax destructor (Clark, 1836) and Cherax quadricarinatus (Von Martens, 1868). Animals 12(5): 543. https://doi.org/10.3390/ani12050543
Lagutkina LYu, Evgrafova EM, Kuzmina EG, Mazlov AM (2021) Hematological and biochemical indicators of Australian red-claw crayfish hemolymph. Bulletin of the Astrakhan State Technical University. Series: Fishing Industry 2: 134–143. https://doi.org/10.24143/2073-5529-2021-2-134-143
Wentao Z, Wen L, Yunlong Z, Danli W, Zhongxiang M, Getao S (2017) Ultrastructural and immunocytochemical analysis of circulating hemocytes from Cherax quadricarinatus (von Martens, 1868). Indian J Anim Res 51(1): 129–134. https://doi.org/10.18805/ijar.v0iOF.6823
Romero X, Turnbull JF, Jiménez R (2000) Ultrastructure and Cytopathology of a Rickettsia-like Organism Causing Systemic Infection in the Redclaw Crayfish, Cherax quadricarinatus (Crustacea: Decapoda), in Ecuador. J Invertebr Pathol 76(2): 95–104. https://doi.org/10.1006/jipa.2000.4952
Sánchez-Salgado JL, Pereyra MA, Agundis C, Calzada-Ruiz M, Kantun-Briceño E, Zenteno E (2019) In vivo administration of LPS and β-glucan generates the expression of a serum lectin and its cellular receptor in Cherax quadricarinatus. Fish and Shellfish Immunol 94: 10–16. https://doi.org/10.1016/j.fsi.2019.08.061
Wu D-L, Liu Z-Q, Huang Y-H, Lv W-W, Chen M-H, Li Y-M, Zhao Y-L (2018) Effects of cold acclimation on the survival, feeding rate, and non-specific immune responses of the freshwater red claw crayfish (Cherax quadricarinatus). Aquacult Internat 26(2): 557–567. https://doi.org/10.1007/s10499-018-0236-4
Paterson BD, Spanogle PT, Davidson GW, Hosking W, Nottingam S, Jussila J, Evans LH (2005) Prediction survival of western rock lobster Panuluris cygnus, using discriminant analysis of hemolymph parameters taken immediately following simulated handling treatments. New Zealand J Marine Freshwat Res 39(5): 1129–1143. https://doi.org/10.1080/00288330.2005.9517380
Lin X, Söderhäll I (2011) Crustacean hematopoiesis and the astakine cytokines. Blood 117(24): 6417–6424. https://doi.org/10.1182/blood-2010-11-320614
Sukhachev AN, Dyachkov IS, Romanyuk DS, Kumeyko VV, Sinitsina VF, Korolkova ED, Kharazova AD, Polevshchikov AV (2013) Morphological analysis of hemocytes of ascidian Halocynthia aurantium. Cell Tiss Biol (Tsitologiya) 55(12): 901–906.
Ghiretti-Magaldi A, Milanesi C, Tognon G (1977) Hemopoiesis in crustacea decapoda: origin and evolution of hemocytes and cyanocytes of Carcinus maenas. Cell Different 6(3–4): 167–186. https://doi.org/10.1016/0045-6039(77)90014-8
Martynova MG, Bystrova OA, Parfenov VN (2008) Synthesis of nucleic acids and localization of atrial natriuretic peptide in the crayfish haemocytes. Cell Tiss Biol (Tsitologiya) 50(3): 243–248. (In Russ).
Van de Braak CBT, Botterblom MHA, Liu W, Taverne N, van der Knaap WPW, Rombout JHWM (2002) The role of the haematopoietic tissue in haemocyte production and maturation in the black tiger shrimp (Penaeus monodon). Fish and Shellfish Immunol 12(3): 253–272. https://doi.org/10.1006/fsim.2001.0369
Ivanov AA, Pronina GI, Koryagina NYu (2021) Physiology of hydrobionts. Lan, SPB. (In Russ).
Xu X, Duan H, Shi Y, Xie S, Song Z, Jin S, Li F, Xiang J (2018) Development of a primary culture system for haematopoietic tissue cells from Cherax quadricarinatus and an exploration of transfection methods. Develop and Comparat Immunol 88: 45–54. https://doi.org/10.1016/j.dci.2018.07.006
Pronina GI, Koryagina NYu (2015) Reference values of physiological and immunological parameters of hydrobionts of different species. Bulletin of the Astrakhan State Technical University; Series: Fisheries 4: 103–108. (In Russ).
Liu YT, Chang CI, Hseu JR, Liu KF, Tsai JM (2013) Immune responses of prophenoloxidase and cytosolic manganese superoxide dismutase in the freshwater crayfish Cherax quadricarinatus against a virus and bacterium. Molec Immunol 56(1–2): 72–80. https://doi.org/10.1016/j.molimm.2013.03.023
Bone JWP, Renshaw GMC, Furse JM, Wild CH (2014) Using biochemical markers to assess the effects of imposed temperature stress on freshwater decapod crustaceans: Cherax quadricarinatus as a test case. J Compar Physiol B 185(3): 291–301. https://doi.org/10.1007/s00360-014-0883-3
Author information
Authors and Affiliations
Contributions
D.N.S.—conceptualization, experimental design, data collection and processing, writing and editing the manuscript; D.V.Sh.—data processing, writing and editing the text.
Corresponding author
Ethics declarations
COMPLIANCE WITH ETHICAL STANDARDS
All applicable international principles on the use of laboratory animals were observed during the study. In view of the work with invertebrates, the ethical standards formulated in the Universal Declaration of Animal Rights were not violated.
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest.
Additional information
Translated by A. Polyanovsky
Russian Text © The Author(s), 2022, published in Zhurnal Evolyutsionnoi Biokhimii i Fiziologii, 2022, Vol. 58, No. 6, pp. 507–519https://doi.org/10.31857/S0044452922060109.
Rights and permissions
About this article
Cite this article
Skafar, D.N., Shumeiko, D.V. Hemocytes of the Australian Red Claw Crayfish (Cherax quadricarinatus): Morphology and Hemogram. J Evol Biochem Phys 58, 1730–1743 (2022). https://doi.org/10.1134/S0022093022060060
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0022093022060060