Abstract
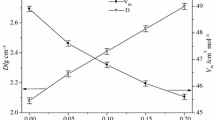
The thermal properties (glass transformation, T g, and softening, T s, temperatures), the crystalline phases formed during heating in a differential thermal analysis (DTA) apparatus, the kinetic parameters and the mechanism of the devitrification process, of glasses of the system diopside-wollastonite were investigated. The substitution of CaO by MgO induces an increase in T g and the crystal growth activation energy, E c; this is probably linked to the greater coordination number of Caz+ ions with respect to the Mg2+ ions. The substitution of CaO by MgO lowers the nucleation rates of the diopside phase; wollastonite solid solution nuclei form whose growth appears to leave a glassy matrix in which diopside formation is inhibited. Only surface nucleation was observed, but, in finely powdered samples, which soften and efficiently sinter before devitrifying, surface nuclei behave as bulk nuclei. When bulk crystallization occurs, the Avrami parameter m was found to be 2 for all glasses, except the diopside one, for which m=3.
Similar content being viewed by others
References
L. L. Hench, J. Am. Ceram. Soc. 74 (1991) 1487.
T. Kokubo, Bol. Soc. Esp. Ceram. Vid. (Proc. XVI International Congress on Glass, Vol. I) 31-C1 (1992) 119.
Z. Strnad, “Glass-Ceramic Materials” (Elsevier, Amsterdam, 1986) p. 110.
E. M. Levin, C. R. Robins and H. F. McMurdie “Phase diagrams for Ceramists” (The American Ceramics Society, Columbus, OH, 1964) P. 211.
K. Matusita and S. Sakka, Bull. Inst. Chem. Res. Kyoto Univ. 59 (1981) 159.
D. R. MacFarlane, M. Matecki and M. Poulain, J. Non-Cryst. Solids 64 (1984) 351.
P. G. Boswell, J. Thermal Anal. 18 (1980) 353.
H. J. Borchardt and F. Daniels, J. Am. Chem. Soc. 79 (1957) 41.
K. Akita and M. Kase, J. Phys. Chem. 72 (1968) 906.
F. O. Piloyan, I. V. Ryabchica and O. S. Novikova, Nature 212 (1966) 1229.
N. H. Ray, J. Non-Cryst. Solids 15 (1974) 423.
H. Rawson, “Inorganic Glass Forming Systems” (Academic Press, London, New York, 1967) p. 24.
A. Costantini, F. Branda and A. Buri, J. Eur. Ceram. Soc., in press.
F. Branda, A. Costantini and A. Buri, Thermochim. Acta 217 (1993) 207.
F. Branda, A. Costantini, A. Buri and A. Tomasi, J. Thermal Anal. 41 (1994) 1979.
A. N. Winchell and H. Winchell, “The microscopical characters of artificial inorganic substances: optical properties of artificial minerals” (Academic Press, New York, London, 1964) p. 291.
E. D. Zanotto, J. Non-Cryst. Solids 130 (1991) 217.
K. Maeda, E. Ichikura, Y. Nakao and S. Ito, Bol. Soc. Esp. Ceram. Vid. (Proc. XVI International Congress on Glass, Vol. 5) 31-C (1992) 15.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Costantini, A., Branda, F. & Buri, A. Thermal properties and devitrification behaviour of (1+x)CaO·(1−x)MgO·2SiO2 glasses. Journal of Materials Science 30, 1561–1564 (1995). https://doi.org/10.1007/BF00375265
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF00375265