Summary
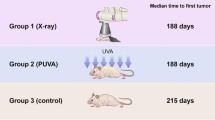
The carcinogenic effect of three UVA tanning sources was studied in lightly pigmented hairless mice. The three tanning sources (Bellarium-S SA-1-12, Philips TL 09R and Philips TL 10R) have different emission spectra, and emit different amounts of UVB. Radiation from the tanning sources was administered for 20 min/day, 5 day/week in daily doses equivalent to those used in suntan salons. The radiation was given alone or after 12 weeks of exposure to solar-simulated UV radiation (SOLAR UV) (10min/day, 4 day/week; daily dose, 19.5 kJ/m2 UVA and 3.9 kJ/m2 UVB). Irradiation with Bellarium-S SA-1-12 for 47 weeks and Philips TL 09R for 74 weeks induced skin tumours in 20/20 and 13/20 of the animals, respectively. When irradiation with Bellarium-S SA-1-12 and Philips TL 09R was administered after 12 weeks of SOLAR-UV exposure, a strong enhancement of SOLAR-UV-induced photocarcinogenesis was observed (p<0.001). Irradiation with Philips TL 10R was only slightly carcinogenic, and during 85 weeks of irradiation only one skin tumor appeared in a group of 20 mice. However, when irradiation with Philips TL 10R was administered after 12 weeks of exposure to SOLAR UV, an enhancement of SOLAR-UV-induced carcinogenesis was observed (p<0.001). Our results suggest that the hazards of exposure to commercial tanning devices are increased when they are used after a period of natural sun exposure. Even tanning sources with a low carcinogenic potential are able to increase SOLAR-UV-induced carcinogenesis significantly.
Similar content being viewed by others
References
Bech-Thomsen N, Wulf HC, Poulsen T, Lundgren K (1988) Pretreatment with long-wave ultraviolet light inhibits ultraviolet-induced skin tumor development in hairless mice. Arch Dermatol 124: 1215–1218
Bech-Thomsen N, Wulf HC, Poulsen T, Christensen FG, Lundgren K (1991) Photocarcinogenesis in hairless mice induced by ultraviolet A tanning devices with or without subsequent solar-simulated ultraviolet irradiation. Photodermatol Photoimmunol Photomed 8: 139–145
Cole CA, Davies RE, Forbes PD, Aloisio LD (1983) Comparison of action spectra for acute cutaneous responses to ultraviolet radiation: man vs albino mice. Photochem Photobiol 37: 623–631
Cole CA, Forbes D, Davies RE (1986) An action spectrum for UV photocarcinogenesis. Photochem Photobiol 43: 275–284
De Gruijl FR, van der Meer JB, van der Leun JC (1983) Dose-time dependency of tumor formation by chronic UV exposure. Photochem Photobiol 37: 53–62
Diffey BL (1987) Analysis of the risk of skin cancer from sunlight and solaria in subjects living in northern Europe. Photodermatol 4: 118–126
Forbes PD, Davies RE, Urbach F (1978) Experimental ultraviolet photocarcinogenesis: Wavelength interactions and time-dose relationships. NCI Monogr 50: 31–38
McKinlay AF, Diffey BL (1987) A reference action spectrum for ultraviolet induced eryhtema in human skin. CIE J 6: 17–22
Parrish JA, Jaenicke KF, Anderson RR (1982) Erythema and melanogenesis action spectra of normal human skin. Photochem Photobiol 36: 187–191
Peto R, Pike MC, Day NE et al. (1980) Annex to international agency for research on cancer supplement 2, IARC Monographs on the evaluation of the carcinogenic risk of chemicals to humans. IARC, Lyon, pp 311–426
Rivers JK, Norris PG, Murphy GM et al. (1989) UVA sunbeds: tanning, photoprotection, acute adverse effects and immunological changes. Br J Dermatol 120: 767–777
Romerdahl CA, Kriepke ML (1988) Role of helper T-lymphocytes in rejection of UV-induced murine skin cancers. Cancer Res 48: 2325–2328
Roza L, Baan RA, Leun JC van der, Kligman L, Young AR (1989) UVA hazards in skin associated with the use of tanning equipment. J Photochem Photobiol/[B] 3: 281–287
Slaper H (1987) Skin cancer and UV exposure: investigations on the estimations of risks. PhD thesis, Utrecht, pp 117–141
Staberg B, Wulf HC, Poulsen T, Klemp P, Brodthagen H (1983) Carcinogenic effect of sequential artificial sunlight and UVA irradiation in hairless mice. Arch Dermatol 119: 641–643
Staberg B, Wulf HC, Klemp P, Poulsen T, Brodthagen H (1983) The carcinogenic effect of UVA irradiation. J Invest Dermatol 81: 517–519
Wulf HC, Poulsen T, Brodthagen H, Hou-Jensen K (1982) Sunscreens for delay of ultraviolet induction of skin tumors. J Am Acad Dermatol 7: 194–202
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bech-Thomsen, N., Poulsen, T., Christensen, F.G. et al. UVA tanning devices interact with solar-simulated UV radiation in skin tumor development in hairless mice. Arch Dermatol Res 284, 353–357 (1992). https://doi.org/10.1007/BF00372039
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00372039