Abstract
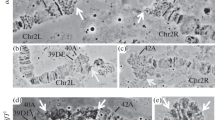
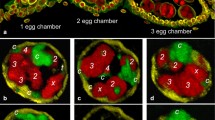
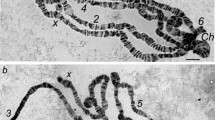
The banding pattern of the polytene chromosomes of the ovarian pseudonurse cells (PNC) of the Drosophila melanogaster otu 1 mutant were compared with larval salivary gland (SG) polytene chromosomes. The X chromosome was studied and no significant differences were found in the banding pattern between these functionally very different tissues. Most of the differences result from differential puffing activity. In situ hybridisation with five different DNA probes located along the X chromosome was used to cross-check the results obtained by morphological mapping. The constrictions present in the SG chromosomes were found to be absent in the germ line derived PNC chromosomes. There are prominent puffs in the PNC chromosomes at certain locations where genes known to be transcriptionally active in the germ line reside. This suggests that at least some of the genes active in the wild-type nurse cells may also be active in the PNC cells.
Similar content being viewed by others
References
Ashburner M (1989) Drosophila: A laboratory handbook. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York
Ashburner M (1991) Drosophila genetic maps. Drosophila Inf Serv 69: 1–399
Ashburner M, Berendes HD (1978) Puffing of polytene chromosomes. In: Ashburner M, Wright TRF (eds) The genetics and biology of Drosophila, vol 2b. Academic Press, London New York, pp 315–395
Bellen HJ, Gregory BK, Olsson CL, Kiger JA (1987) Two Drosophila learning mutants, dunce and rutabaga, provide evidence of a material role for cAMP on embryogenesis. Dev Biol 121: 432–444
Berendes HD (1965) Salivary gland function and chromosomal puffing patterns in Drosophila hydei. Chromosoma 17: 35–77
Bridges CB (1938) A revised map of the salivary gland X chromosome of Drosophila melanogaster. J Hered 29: 11–13
Chen C-N, Malone T, Beckendorf SK, Davis RL (1987) At least two genes reside within a large intron of the dunce gene of Drosophila. Nature 329: 721–724
Danilevskaya ON, Kurenova EV, Pavlova MN, Bebehor DV, Link A, Koga A, Vellek A, Hartl DL (1991) He-T family DNA sequences in the Y chromosome of Drosophila melanogaster share homology with the X-linked Stellate genes. Chromosoma 100: 118–124
DeCamillis M, Cheng N, Pierre D, Brock HW (1992) The polyhomeotic gene of Drosophila encodes a chromatin protein that shares polytene chromosome-binding sites with Polycomb. Genes Dev 6: 223–232
de Frutos R, Kimura K, Peterson K (1990) In situ hybridization of Drosophila polytene chromosomes with digoxigenin dUTP labeled probes. Methods Mol Cell Biol 2: 32–36
Eanes WF, Hey J (1986) In vivo function of rare G6pd variants from natural populatons of Drosophila melanogaster. Genetics 113: 679–693
Ephrussi B, Beadle GW (1936) A technique of transplantation for Drosophila. Am Nat 70: 218–225
Galanopoulos VK, Orr W, Szabad J, Kafatos FC (1989) Genetic analysis of chorion formation in Drosophila melanogaster. I. The effects of one somatic-specific and seven germ-line-specific mutations. Dev Genet 10: 87–97
Ganguly R, Ganguly N, Manning JE (1985) Isolation and characterization of the glucose-6-phosphate dehydrogenase gene of Drosophila melanogaster. Gene 35: 91–101
Gans M, Audit C, Masson M (1975) Isolation and characterization of sex-linked female-sterile mutants in Drosophila melanogaster. Genetics 81: 683–704
Haenlin M, Roos C, Cassab A, Mohier E (1988) Oocyte-specific transcription of fs(1)K10: a Drosophila gene affecting dorsalventral polarity. EMBO J 6: 801–807
Hartl DL (1992) Genome map of Drosophila based on yeast artificial chromosomes. In: Davies EK, Tilghman DM (eds) Genome analysis, vol 4. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York, pp 39–69
Heino TI (1989) Polytene chromosomes from ovarian pseudonurse cells of the Drosophila melanogaster otu mutant I. Photographic map of chromosome 3. Chromosoma 97: 363–373
Hochstrasser M (1987) Chromosome structure in four wild-type polytene tissues of Drosophila melanogaster. The 87A and 87C heat shock loci are induced unequally in the midgut in a manner dependent of the growth temperature. Chromosoma 95: 197–208
Holden JJ, Ashburner M (1978) Patterns of puffing activity in the salivary glands of Drosophila. IX. The salivary and prothoracic gland chromosomes of a dominant temperature sensitive lethal of D. melanogaster. Chromosoma 68: 205–227
Hori SH, Akasaka M, Ito H, Hanaoka T, Tanda S, Ohtsuka E, Miura K, Takahashi T, Tang JJN (1985) Cloning of the glucose-6-phosphate dehydrogenase gene of Drosophila melanogaster using 17-base oligonucleotide mixtures as probes. Jpn J Genet 60: 455–463
Itoh M, Iwabuchi M, Yoshida K, Hori SH (1989) Four tandem defective P elements associated with positive regulation of the Drosophila melanogaster glucose-6-phosphate dehydrogenase gene. Biochem Genet 27: 699–718
Kafatos FC, Louis C, Savakis C, Glover DM, Ashburner M, Link AJ, Sidén-Kiamos I, Saunders RDC (1991) Integrated maps of the Drosophila genome: progress and prospects. Trends Genet 7: 155–161
King RC, Büning J (1985) The origin and functioning of insect oocytes and nurse cells. In: Kerkut GA, Gilbert LI (eds) Comprehensive insect physiology, biochemistry and pharmacology, vol 1. Pergamon Press, Oxford, pp 37–82
King RC, Bahns M, Horowitz R, Larramendi P (1978) A mutation that effects female and male germ cells differentially in Drosophila melanogaster Meigen (Diptera: Drosophilidae). Int J Insect Morphol Embryol 7: 359–375
King RC, Riley SF, Cassidy JD, White PE, Paik YK (1981) Giant polytene chromosomes from the ovaries of a Drosophila mutant. Science 212: 441–443
King RC, Mohler D, Riley SF, Storto PD, Nicollazzo PS (1986) Complementation between alleles at the ovarian tumor locus of Drosophila melanogaster. Dev Genet 7: 1–20
Korge G (1977) Larval saliva in Drosophila melanogaster: Production, composition and relationship to chromosome puffs. Dev Biol 58: 339–355
Lamb MM, Laird CD (1987) Three euchromatic DNA sequences under-replicated in polytene chromosomes of Drosophila are localized in constrictions and ectopic fibers. Chromosoma 95: 227–235
Lefevre G Jr (1976) A photographic representation and interpretation of the polytene chromosomes of Drosophila melanogaster salivary glands. In: Ashburner M, Novitsky E (eds) Genetics and biology of Drosophila, vol 1 a. Academic Press, London, pp 31–66
Lindsley DL, Zimm G (1985) The genome of Drosophila melanogaster. Part 1: Genes A-K. Drosophila Inf Serv 62: 1–227
Lindsley DL, Zimm G (1990) The genome of Drosophila melanogaster. Part 4: Genes L-Z. Drosophila Inf Serv 68: 1–382
Livak KJ (1984) Organization and mapping of a sequence on the Drosophila melanogaster X and Y chromosomes that is transcribed during spermatogenesis. Genetics 107: 611–634
Livak KJ (1990) Detailed structure of the Drosophila melanogaster Stellate genes and their transcripts. Genetics 124: 303–316
Lovett JA, Kaufman TC, Mahowald AP (1980) A locus on the X chromosome apparently controlled by the Y chromosome during spermatogenesis in Drosophila melanogaster. Eur J Cell Biol 22: 49
Mével-Ninio M, Terracol R, Kafatos FC (1991) The ovo gene of Drosophila encodes a zinc finger protein required for female germ line development. EMBO J 10: 2259–2266
O'Kane CJ, Gehring WJ (1987) Detection in situ of genomic regulatory elements in Drosophila. Proc Natl Acad Sci USA 84: 9123–9127
Oliver B, Perrimon N, Mahowald AP (1987) The ovo locus is required for sex-specific germ line maintenance in Drosophila. Genes Dev 1: 913–923
Orr WC, Galanopoulos VK, Romano CP, Kafatos FC (1989) A female sterile screen of the Drosophila melanogaster X chromosome using hybrid dysgenesis: Identification and characterization of egg morphology mutants. Genetics 122: 847–858
Paro R (1990) Imprinting a determined state into the chromatin of Drosophila. Trends Genet 6: 416–421
Perrimon N, Engstrom L, Mahowald AP (1984) Developmental genetics of the 2E-F region of the Drosophila X-chromosome. A region rich in “developmentally important” genes. Genetics 108: 559–572
Pongs O, Kecskemethy N, Müller R, Krah-Jentgens I, Baumann A, Kiltz HH, Canal I, Llamazares S, Ferrus A (1988) Shaker encodes a family of putative potassium channel proteins in the nervous system of Drosophila. EMBO J 7: 1087–1096
Qiu Y, Davis RL (1993) Genetic dissection of the learning/memory gene dunce of Drosophila melanogaster. Genes Dev 7: 1447–1458
Redfern CPF (1981) Homologous banding patterns in the polytene chromosomes from larval salivary glands and ovarian nurse cells of Anopheles stephensi Liston (Culicidae). Chromosoma 83: 221–240
Ribbert D (1979) Chromomeres and puffing in experimentally induced polytene chromosomes of Calliphora erythrocephala. Chromosoma 74: 269–298
Richards G (1980) The polytene chromosomes in the fat body nuclei of Drosophila melanogaster. Chromosoma 79: 241–250
Richards G (1985) Polytene chromosomes. In: Kerkut GA, Gilbert LI (eds) Comprehensive insect physiology, biochemistry and pharmacology, vol 2. Pergamon Press, Oxford, pp 255–300
Schalenge F, Turco E, Edström JE, Pirrotta V, Melli M (1981) Microdissection and cloning of DNA from a specific region of Drosophila melanogaster polytene chromosomes. Chromosoma 82: 205–216
Segarra C, Aguadé M (1992) Molecular organization of the X chromosome in different species of the obscura group of Drosophila. Genetics 130: 513–521
Shevelyov YY (1992) Copies of a stellate gene variant are located in the X heterochromatin of Drosophila melanogaster and are probably expressed. Genetics 132: 1033–1037
Sinha P, Mishra A, Lakhotia SC (1987) Chromosomal organization of Drosophila tumours. I. Polytene chromosome organization and DNA synthesis in ovarian pseudonurse cells in otu mutants of D. melanogaster. Chromosoma 95: 108–116
Sorsa V (1988) Chromosome maps of Drosophila, II. CRC Press, Boca Raton, Florida
Spierer A, Spierer P (1984) Similar level of polyteny in bands and interbands of Drosophila giant chromosomes. Nature 307: 176–178
Stalker HD (1954) Banded polytene chromosomes in the ovarian nurse cells of adult Diptera. J Hered 45: 259–264
Steinhauer WR, Walsh RC, Kalfayan LJ (1989) Sequence and structure of the Drosophila melanogaster ovarian tumor gene and generation of an antibody specific for the ovarian tumor protein. Mol Cell Biol 9: 5726–5732
Tanouye MA, Ferrus A, Fujita SC (1981) Abnormal action potentials associated with the Shaker complex locus of Drosophila. Proc Natl Acad Sci USA 78: 6548–6552
Tirronen M, Partanen M, Heino TO, Heino TI, Roos C (1992) Analysis of the Drosophila quit, ovarian tumor and shut down mutants in oocyte differentiation using in situ hybridisation. Mech Dev 40: 113–126
Zacharopolou A, Bourztis K, Kerremans Ph (1991) A comparison of polytene chromosomes in salivary glands and orbital bristle trichogen cells in Ceratitis capitata. Genome 34: 215–219
Zacharopoulou A, Frisardi M, Savakis C, Robinson AS, Tolias P, Konsolaki M, Komitopoulou K, Kafatos FC (1992) The genome of the Mediterranean fruitfly Ceratitis capitata: Localization of molecular markers by in situ hybridization to salivary gland polytene chromosomes. Chromosoma 101: 448–455
Zalokar M, Audit C, Erk I (1975) Developmental defects on female sterile mutants of Drosophila melanogaster. Dev Biol 47: 419–432
Zhimulev IF, Belyaeva ES (1975) 3H-uridine labelling patterns in the Drosophila melanogaster salivary gland chromosomes X, 2R and 3L. Chromosoma 49: 219–231
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Heino, T.I. Polytene chromosomes from ovarian pseudonurse cells of the Drosophila melanogaster otu mutant. Chromosoma 103, 4–15 (1994). https://doi.org/10.1007/BF00364721
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00364721