Abstract
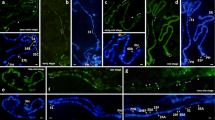
The spatial organization of polytene chromosomes and the association of their pericentromeric regions in the Drosophila melanogaster nurse cells during oogenesis have been examined by 3D-immunofluorescence microscopy. All nurse cell chromosomes are shown to contact the nuclear membrane with their pericentromeric regions, and the X chromosome additionally with its telomeric region. Three morphological types of the associations of the nurse cell chromosome pericentromeric regions in the nuclear space are observed: (1) the pericentromeric regions of chromosomes 2, 3, and 4 contact the nuclear membrane at one pole of the nucleus, while the pericentromeric region of the X chromosome does so at the other pole (morphotype I); (2) the pericentromeric regions of chromosomes X, 2, and 3 are separated from each other in the nuclear space and contact the nuclear membrane (morphotype II); and (3) the pericentromeric region of chromosome 2 contacts the nuclear membrane at one pole of the nucleus, while the pericentromeric regions of chromosomes X, 3, and 4 contact the membrane at the other pole (morphotype III). The author, therefore, proposes that the nurse cell nuclei with morphotype I are prevalent at the early stages of oogenesis, while the nurse cell nuclei with morphotype III are most abundant at the late stages. The dynamics of associations of the pericentromeric chromosome regions in the nuclear space of D. melanogaster ovarian nurse cells in the oogenesis demonstrated that there may be some functional relationship between the 3-D organization of the nurse cell chromosomes and the organization of the nucleolus.





Similar content being viewed by others
References
Aizenshtadt TB, Baranov VS, Borovkov AYu. The current problems in oogenesis. Moscow: Nauka; 1977.
Alcobia I, Dilao R, Parreira L. Spatial associations of centromeres in the nuclei of hematopoietic cells: evidence for cell-type-specific organizational patterns. Blood. 2000;95:1608–15.
Ananiev EV, BarskyVE Ilyin YV, Churikov A. Localization of nucleoli in Drosophila melanogaster polytene chromosomes. Chromosoma (Berl). 1981;81:619–28.
Berr A, Pecinka A, Meister A, Kreth G, Fuchs J, Blattner FR, Lysak MA, Schubert I. Chromosome arrangement and nuclear architecture but not centromeric sequences are conserved between Arabidopsis thaliana and Arabidopsis lyrata. Plant J. 2006;48:771–83.
Boikova TV, Orlando V, Lupo R, Bogachev SS. M/SAR elements of the Bithorax complex of Drosophila melanogaster. Genetika (Mosk). 2005;41:1–12.
Bolzer A, Kreth G, Solovei I, Koehler D, Saracoglu K, Fauth C, Muller S, Eils R, Cremer C, Speicher MR, Cremer T. Three-dimensional maps of all chromosomes in human male fibroblast nuclei and prometaphase rosettes. PLoS Biol. 2005. https://doi.org/10.1371/journal.pbio.0030157.
Branco M, Pombo A. Chromosome organization: new facts, new models. Trends Cell Biol. 2007;17:127–34.
Capell BC, Collins FS. Human laminopathies: nuclei gone genetically awry. Nat Rev Genet. 2006;7:940–52.
Chubb JR, Boyle S, Perry P, Bickmore WA. Chromatin motion is constrained by association with nuclear compartments in human cells. Curr Biol. 2002;12:439–45.
Cremer T, Cremer C. Chromosome territories, nuclear architecture and gene regulation in mammalian cells. Nat Rev Genet. 2001;2:292–301.
Cremer T, Cremer M. Chromosome territories. Cold Spring Harb Perspect Biol. 2010. https://doi.org/10.1101/cshperspect.a003889.
Cremer T, Cremer M, Dietzel S, Muller S, Solovei I, Fakan S. Chromosome territories—a functional nuclear landscape. Curr Opin Cell Biol. 2006;18:307–16.
Croft JA, Bridger JM, Boyle S, Perry P, Teague P, Bickmore WA. Differences in the localization and morphology of chromosomes in the human nucleus. J Cell Biol. 1999;145:1119–31.
Csink AK, Henikoff S. Large-scale chromosomal movements during interphase progression in Drosophila. J Cell Biol. 1998;143:13–22.
Dapples CC, King RC. The development of the nucleolus of the ovarian nurse cell of Drosophila melanogaster. Zellforsch Mikrosk Anat. 1970;103:34–47.
De Boni U. The interphase nucleus as a dynamic structure. Int Rev Cytol. 1994;150:149–71.
De Boni U, Mintz AH. Curvilinear, three-dimensional motion of chromatin domains and nucleoli in neuronal interphase nuclei. Science. 1986;234:863–6.
Dehghani H, Dellaire G, Bazett-Jones DP. Organization of chromatin in the interphase mammalian cell. Micron. 2005;36:95–108.
Dej KJ, Spradling AC. Theendocycle controls nurse cell polytene chromosome structure during Drosophila oogenesis. Development. 1999;126:293–303.
Del Prete S, Arpon J, Sakai K, Andrey P, Gaudin V. Nuclear architecture and chromatin dynamics in interphase nuclei of Arabidopsis thaliana. Cytogenet Genome Res. 2014. https://doi.org/10.1159/000363724.
Fransz P, De Jong JH, Lysak M, Castiglione MR, Schubert I. Interphase chromosomes in Arabidopsis are organized as well defined chromocenters from which euchromatin loops emanate. Proc Natl Acad Sci USA. 2002;99:14584–9.
Fritz A, Barutcu AR, Martin-Buley L, van Wijnen AJ, Zaidi SK, Imbalzano AN, Lian JB, Stein JL, Stein GS. Chromosomes at work: organization of chromosome territories in the interphase nucleus. J Cell Biochem. 2016;117:9–19.
Gavrilov AA, Razin SV. Compartmentalization of the cell nucleus and spatial organization of the genome. Mol Biol. 2015;49:26–45.
Gavrilov AA, Razin SV, Iarovaia OV. C-methods to study 3D organization of the eukaryotic genome. Biopolym Cell. 2012;28:245–51.
Getzenberg RH, Pienta KJ, Ward WS, Coffey DS. Nuclear structure and the three-dimensional organization of DNA. J Cell Biochem. 1991;47:289–99.
Glazkov MV. A loop-domain arrangement of the genes in eukaryotic chromosomes. Mol Biol. 1995;29:965–82.
Gorkin DU, Leung D, Ren B. The 3D genome in transcriptional regulation and pluripotency. Stem Cell. 2014;14:762–75.
Glazkov MV. Association of chromosomes with the nuclear membrane and the orderliness of the spatial organization of genetic material in the interphase nucleus. Tsitol Genet. 1999;33:79–88.
Guo T, Fang Y. Functional organization and dynamics of the cell nucleus. Front Plant Sci. 2014. https://doi.org/10.3389/fpls.2014.00378.
Gushchanskaya ES, Gavrilov AA, Razin SV. Spatial organization of interphase chromosomes and the role of chromatin fiber dynamics in the positioning of genome elements. Mol Biol. 2014;48:386–94.
Holmquist G. Transcription rates of individual polytene chromosome bands: effects of gene dose and sex in Drosophila. Chromosoma (Berl). 1972;36:413–52.
Kiknadze II, Istomina AG, Salova TA. Functional morphology of the polytene chromosomes of the Chironomus pilicornis F. from the water bodies of cryolithozone. Tsitologiya. 2002;44:89–95.
Kokhanenko AA, Anan’ina TV, Stegnii VN. Intranuclear dynamics of chromosome 6 in nurse cells of Calliphoraerythrocephala Mg. (Diptera: Calliphoridae). Russ J Genet. 2010;46:1045–7.
Kokhanenko AA, Anan’ina TV, Stegniy VN. The changes in chromosome 6 spatial organization during chromatin polytenization in the Calliphora erythrocephala Mg. (Diptera: Calliphoridae) nurse cells. Protoplasma. 2013;250:141–9.
Kokhanenko AA, Anan’ina TV, Stegniy VN. Localization of rRNA genes in the nuclear space of Calliphoraerythrocephala Mg. nurse cells during polytenization. Protoplasma. 2014;251:93–101.
Kubben N, Adriaens M, Meuleman W, Voncken JW, van Steensel B, Misteli T. Mapping of lamin A- and progerin-interacting genome regions. Chromosoma. 2012;121:447–64.
Kulichkov VA, Zhimulev IF. Analysis of spatial organization of the Drosophila melanogaster genome based on the data on ectopic conjugation of polytene chromosomes. Genetika (Mosk). 1976;12:81–9.
Lefevre G. A photographic representation and interpretation of the polytene chromosomes of Drosophila melanogaster salivary glands. In: Ashburner M, Novitski E, editors. The genetics and biology of Drosophila, vol. 1a. New York: Academic Press; 1976. p. 31–66.
Mannuelidis L. Indications of centromere movement during interphase and differentiation. Ann N Y Acad Sci. 1985;450:205–21.
Marshall WF, Sedat JW. Nuclear architecture. Res Probl Cell Differ. 1999;25:283–301.
Marshall WF, Straight A, Marko JF, Demburg AF, Swedlow JR, Murray A, Belmont A, Agard DA, Sedat JW. Interphase chromosomes undergo constrained diffusional motion in living cells. Curr Biol. 1997;7:930–9.
Meaburn KJ, Misteli T. Cell biology: chromosome territories. Nature. 2007;445:379–781.
Mecheva IS, Semionov EP. Localization of ribosomal DNA insertion elements in polytene chromosomes of Drosophila simulans, Drosophila mauritiana and their interspecific hybrids. Genetica. 1992;85:223–9.
Nemeth A, Langst G. Genome organization in and around the nucleolus. Trends Genet. 2011;27:149–56.
Pontvianne F, Carpentier M-C, Durut N, Pavlistova V, Jaske K, Schorova S, Parrinello H, Rohmer M, Pikaard CS, Fojtova M, Fajkus J, Saez-Vasquez J. Identification of nucleolus-associated chromatin domains reveals a role for the nucleolus in 3D organization of the A. thaliana genome. Cell Rep. 2016;16:1574–87.
Razin SV. Spatial organization of the eukaryotic genome and the operation of epigenetic mechanisms. Genetika (Mosk). 2006;42:1605–14.
Scaffidi P, Misteli T. Lamin A-dependent nuclear defects in human aging. Science. 2006;312:1059–63.
Schubert V, Berr A, Meister A. Interphase chromatin organization in Arabidopsis nuclei: constraints versus randomness. Chromosoma. 2012;121:369–87.
Schwartz M, Hakim O. 3D view of chromosomes, DNA damage, and translocations. Curr Opin Genet Dev. 2014;25:118–25.
Sharakhov IV, Wasserlauf IE, Stegnii VN. Features of polytene chromosome attachment to the nuclear envelope of ovarian pseudonurse cells in Drosophila melanogaster. Russ J Genet. 1997;33:139–44.
Shchapova AI. Spatial organization of chromosomes in the eukaryotic cell nucleus of various plant and animal species. Vestn VOGiS. 2010;14:612–21.
Shelkovnikova TA, Wasserlauf IE, Stegniy VN. The changes in nuclear architecture during the ovarian development in the Drosophila subgroup melanogaster. Vestn Tomsk Gos Univ. 2007;301:222–6.
Solovei I, Cremer M. 3D-FISH on cultured cells combined with immunostaining. Methods Mol Biol. 2010;659:117–26.
Stegniy VN. Reorganization of the structure of the interphase nuclei in the ontogeny and phylogeny of malaria mosquitoes. Dokl Ross Akad Nauk. 1979;249:1231–4.
Stegniy VN. Architectonics of the genome, systemic mutations, and evolution. Novosibirsk: Novosibirsk State University; 1993.
Stegniy VN. The evolutionary significance of chromosome architectonics as a form of the epigenetic control of ontogenesis and phylogenesis in eukaryotes. Genetika (Mosk). 2006;42:1215–24.
Stegniy VN, Sharakhova MV. Systemic restructuring of the architectonics of polytene chromosomes in the ontogeny and phylogeny of malaria mosquitoes. Specific structural features of the zones of chromosome contact with the nuclear envelope. Genetika (Mosk). 1991;27:828–35.
Stegniy VN, Wasserlauf IE. Interspecific differences in the co-orientation of primary polytene chromosomes of the Drosophila melanogaster, D. simulans, and D. mauritiana. Genetika (Mosk). 1991;27:1169–73.
Wasserlauf IE. Thedynamics of chromosome orientation in the ovarian nurse cell nuclei in closely related species of the D. melanogaster subgroup and D. virilis group. Vestn Tomsk Gos Univ. 2008;313:205–14.
Xue F, Cooley L. Kelch encodes a component of intercellular bridges in Drosophila egg chambers. Cell. 1993;72:681–93.
Zimmer C, Fabre E. Principles of chromosomal organization: lessons from yeast. J Cell Biol. 2011;192:723–33.
Zink D, Cremer T. Chromosome dynamics in nuclei of living cells. Curr Biol. 1998;8:321–4.
Zuleger N, Robson MI, Schirmer EC. The nuclear envelope as a chromatin organizer. Nucleus. 2011;2:339–49.
Acknowledgements
This research work was funded by the Tomsk State University competitiveness improvement programme. We thank Mr. Ian Barrett for editing the manuscript.
Author information
Authors and Affiliations
Contributions
IEW: analysis of the results, drawing up illustrations for the article, writing the article; KEU: setting the experiment, analyzing the results, writing the article; AKS: statistical processing of the results, analysis of the results; VNS: study design, interpretation of results, critical revision and finalization of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standards
The authors of this manuscript declare that all experiments comply with the current laws of the country in which they were performed.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wasserlauf, I.E., Usov, K.E., Sibataev, A.K. et al. Dynamics of the spatial orientation of the pericentromeric heterochromatin regions in the polytene chromosomes of ovarian nurse cells in the Drosophila melanogaster (Diptera: Drosophilidae) oogenesis. Nucleus 63, 7–15 (2020). https://doi.org/10.1007/s13237-019-00275-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13237-019-00275-2