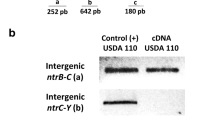
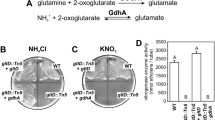
Summary
Bradyrhizobial strain BTAi 1 nodulates both stems and roots of Aeschynomene spp. Previous work has shown that it contains bacteriochlorophyll a and forms photosynthetic reaction centers, and has provided indirect evidence of photosynthesis by bacteroids within stem nodules. Here we report physiological and biochemical characteristics of BTAi 1 ex planta, which also suggest the presence of photosynthetic activity. Light-stimulated uptake of 14CO2 by BTAi 1 was detected at all stages of growth. Inhibitors of photosynthesis, 1,10-orthophenanthroline and 3-(3,4-dichlorophenyl)-1,1-dimethylurea (DCMU), and the uncoupler NH4Cl, immediately suppressed light-driven 14CO2 uptake and increased O2 uptake. BTAi 1 is strictly aerobic and was unable to grow without organic C even in the light; also, it was unable to grow chemoautotrophically in an atmosphere enriched with H2 and CO2. In micro-aerobic conditions, strain BTAi 1 expressed acetylene reducing activity ex planta in an N-free medium. The highest rates of light-stimulated 14CO2 uptake and acetylene-reducing activity occurred during the exponential and early stationary phases of growth. Acetylene-reducing rates at a low glucose concentration were increased following a light-dark cycle in comparison with continuous dark conditions.
Similar content being viewed by others
References
Alazard D (1990) Nitrogen fixation in pure culture by rhizobia isolated from stem nodules of tropical Aeschynomene species. FEMS Microb Lett 68:177–182
Arora N (1954) Morphological development of the root and stem nodules of Aeschynomene indica L. Phytomorphology 4:211–216
Bergersen FJ, Turner GH, Gibson AH, Dudman WF (1976) Nitrogenase activity and respiration of cultures of Rhizobium spp. with special reference to concentration of dissolved oxygen. Biochim Biophys Acta 444:164–174
Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Chakrabarti SK, Mishra AK, Chakrabartty PK (1986) A note on physiological characteristics and genetic relatedness of a fast-growing Rhizobium from stem nodule of Aeschynomene aspera L. J Appl Bacteriol 60:463–468
Cohen-Bazire G, Sistrom WR, Stanier PY (1957) Kinetic studies of pigment synthesis by non-sulphur purple bacteria. J Cell Comp Physiol 49:25–35
Dreyfus BL, Dommergues YR (1981) Nitrogen-fixing nodules induced by Rhizobium on the stem of the tropical legume Sesbania rostrata. FEMS Microbiol Lett 10:313–317
Dreyfus BL, Elmerich C, Dommergues YR (1983) Free-living Rhizobium strain able to grow on N2 as the sole nitrogen source. Appl Environ Microb 45:711–713
Dreyfus B, Garcia JL, Gillis M (1988) Characterization of Azorhizobium caulinodans gen. nov., sp. nov., a stem-nodulating nitrogen fixing bacterium isolated from Sesbania rostrata. Int J Syst Bacteriol 38:89–98
Eaglesham ARJ, Szalay AA (1983) Aerial stem nodules on Aeschynomene spp. Plant Sci Lett 29:265–272
Eaglesham ARJ, Hassouna S, Seegers R (1983) Effect of fertilizer N on N2-fixation by cowpea and soybean. Agron J 75:61–66
Eaglesham ARJ, Ellis JM, Evans WR, Fleischman DE, Hungria M, Hardy RWF (1990) First photosynthetic nitrogen-fixing Rhizobium: Characteristics. In: Gresshoff PM, Roth LE, Stacey G, Newton WE (eds) Nitrogen fixation: Achievements and objectives. Chapman and Hall, New York, pp 805–811
Evans RJ, Fleischman DE, Calvert HE, Pyati PV, Alter GM, Subba Rao NS (1990) Bacteriochlorophyll and photosynthetic reaction centers in Rhizobium strain BTAi 1. Appl Environ Microb 56:3445–3449
Fernald ML (1950) Gray's manual of botany. American Book Co, New York
Gibson AH, Roper MM, Halsall DM (1988) Nitrogen fixation not associated with legumes. In: Wilson JR (ed) Advances in nitrogen cycling in agricultural ecosystems. CAB International, Wallingford, pp 66–68
Hagerup O (1928) En hygrofil bælgplante (Aeschynomene aspera L.). med bakteriknolde paa stængelen. Dan Bot Ark 15:1–9
Hanus FJ, Maier ARJ, Evans HJ (1979) Autotrophic growth of H2-uptake-positive strain of Rhizobium japonicum in an atmosphere supplied with hydrogen. Proc Natl Acad Sci USA 76:1788–1792
Harashima K, Shiba T, Totsuka T, Simidu U, Taga N (1978) Occurrence of bacteriochlorophyll a in a strain of an aerobic heterotrophic bacterium. Agric Biol Chem 42:1627–1628
Harashima K, Hayasaki J-I, Ikari T, Shiba T (1980) O2-stimulated synthesis of bacteriochlorophyll and carotenoids in marine bacteria. Plant Cell Physiol 21:1283–1294
Harashima K, Nakagawa M, Murata N (1982) Photochemical activities of bacteriochlorophyll in aerobically grown cells of aerobic heterotrophs, Erythrobacter species (OCh 114) and Erythrobacter longus (OCh 101). Plant Cell Physiol 23:185–193
Hennecke H, Kaluza K, Thony B, Fuhrmann M, Ludwig W, Stackebrandt E (1985) Concurrent evolution of nitrogenase genes and 16S rRNA in Rhizobium species and other nitrogen fixing bacteria. Arch Microbiol 142:342–348
Hungria M, Eaglesham ARJ, Hardy RWF (1992) Physiological comparisons of root and stem nodules of Aeschynomene scabra and Sesbania rostrata. Plant and Soil 139:7–13
Izawa S, Good NE (1972) Inhibition of photosynthetic electron transport and photophosphorylation. In: San Pietro A (ed) Photosynthesis and nitrogen fixation. Academic Press, New York, pp 355–377
Keister DL (1978) Respiration vs. photosynthesis. In: Clayton RK, Sistron WR (eds) Photosynthetic bacteria. Plenum Press, New York, pp 849–856
Kurz WG, LaRue TA (1975) Nitrogenase activity in rhizobia in absence of plant host. Science 256:407–408
Lascelles J (1959) Adaptation to form bacteriochlorophyll in Rhodopseudomonas sphaeroides: Changes in activity of enzymes concerned in pyrrole synthesis. Biochem J 72:509–519
McComb JA, Elliot J, Dilworth MJ (1975) Acetylene reduction by Rhizobium in pure culture. Science 256:409–410
Miles AA, Misra SS (1938) The estimation of the bactericidal power of blood. J Hyg 38:732–749
Nishimura Y, Shimadzu M, Iizuka H (1981) Bacteriochlorophyll formation in radiation-resistant Pseudomonas radiora. J Gen Appl Microbiol 27:427–430
Okamura K, Mitsumori F, Ito O, Takamiya K-I, Nishimura M (1986) Photophosphorylation and oxidative phosphorylation in intact cells and chromatophores of an aerobic photosynthetic bacterium, Erythrobacter sp. strain OCh114. J Bacteriol 168:1142–1146
Pagan JD, Child JJ, Scowcroft WR, Gibson AH (1975) Nitrogen fixation by Rhizobium cultured on a defined medium. Science 256:406–407
Pfening NF (1978) General physiology and ecology of photosynthetic bacteria. In: Clayton RK, Sistrom WR (eds) Photosynthetic bacteria. Plenum Press, New York, pp 3–7
Sato K (1978) Bacteriochlorophyll formation by facultative methylotrophs, Protaminobacter ruber and Pseudomonas AM-1. FEBS Lett 85:207–210
Shiba T (1984) Utilization of light energy by the strictly aerobic bacterium Erythrobacter sp. OCh114. J Gen Appl Microbiol 30:239–244
Shiba T (1991) Roseobacter litoralis gen. nov., sp. nov., and Roseobacter denitrificans sp. nov., aerobic pink-pigmented bacteria which contain bacteriochlorophyll a. Syst Appl Microbiol 14:140–145
Shiba T, Simidu U (1986) Erythrobacter longus gen. nov., sp. nov., an aerobic bacterium which contains bacteriochlorophyll a. Int J Syst Bacteriol 32:211–217
Shiba T, Harashima K (1986) Aerobic photosynthetic bacteria. Microbiol Sci 3:376–378
Shiba T, Simidu U, Taga N (1979a) Distribution of aerobic bacteria which contain bacteriochlorophyll a. Appl Environ Microbiol 38:43–45
Shiba T, Simidu U, Taga N (1979b) Another aerobic bacterium which contains bacteriochlorophyll a. Bull Jpn Soc Sci Fish 45:801
Simpson FB, Maier RJ, Evans HJ (1979) Hydrogen-stimulated CO2 fixation and coordinate induction of hydrogenase and ribulosebisphosphate carboxylase in a H2-uptake positive strain of Rhizobium japonicum. Arch Microbiol 123:1–8
Stewart WDP (1980) Some aspects of structure and function in N2-fixing cyanobacteria. Annu Rev Microbiol 37:497–536
Stowers MD, Eaglesham ARJ (1983) A stem-nodulating Rhizobium with physiological characteristics of both fast and slow growers. J Gen Microbiol 129:3651–3655
Takamiya K, Okamura K (1984) Photochemical activities and photosynthetic ATP formation in membrane preparation from a facultative methylotroph, Protaminobacter ruber strain NR-1. Arch Microbiol 140:21–26
Thore A, Keister DL, San Pietro A (1969) Studies on the respiratory system of aerobically (dark) and anaerobically (light) grown Rhodospirillum rubrum. Arch Microbiol 67:378–396
Vincent JM (1970) A manual for the practical study of root nodule bacteria. Oxford, Blackwell
von Sussenguth K, Beyerle R (1936) Über bakterienknöllchen am spross von Aeschynomene paniculata Willd. Hedwigia 75:234–237
Woese CR (1987) Bacterial evolution. Microbiol Rev 51:221–271
Young JPW, Downer HL, Eardly BD (1991) Phylogeny of the phototropic Rhizobium strain BTAi 1 by polymerase chain reaction based sequencing of a 16S rRNA gene segment. J Bacteriol 173:2271–2277
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hungria, M., Ellis, J.M., Hardy, R.W.F. et al. Light-stimulated 14CO2 uptake and acetylene reduction by bacteriochlorophyll containing stem nodule isolate BTAi 1. Biol Fertil Soils 15, 208–214 (1993). https://doi.org/10.1007/BF00361613
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00361613