Abstract
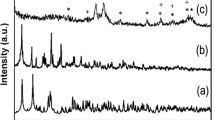
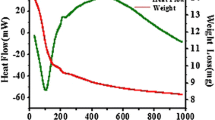
Nanocomposite materials based on silica aerogel hosts have been produced using chemical vapour infiltration/decomposition methods and characterized by X-ray diffraction and electron microscopy. Amorphous tungsten in SiO2 aerogel was formed by the decomposition of W(CO)6 at 250 °C. Alternatively, reaction of this material with sulphur at 700 °C produced needle-shaped WS2 crystals with lengths ranging from 25–230 nm. Reaction of the W/SiO2 composite with anhydrous NH3 formed crystals of δ-WN with diameters of 1–5 nm. Fe(CO)5 is readily absorbed into the silica aerogel, forming an amorphous iron oxide/SiO2 composite after slow oxidation in air. Treatment of this material with additional Fe(CO)5 produced an Fe3O4/SiO2 aerogel composite. Fe3O4 particle sizes were 20–55 nm. After additional heat treatment, this composite exhibited soft ferromagnetic behaviour with a coercivity of ∼170 Oe. Fe9S10 crystals with diameters of 30–90 nm were formed by the reaction of the amorphous iron oxide/SiO2 composite with H2S at 900 °C.
Similar content being viewed by others
References
G. D. Stucky and J. E. MacDougal, Science 247 (1990) 669.
T. Shinjo, J. Phys. Soc. Jpn 21 (1966) 327.
B. Rodmacq, J. Phys. Chem. Solids 45 (1984) 1119.
J. Q. Wang, P. Xiong and G. Xiao, Phys. Rev. B Condens. Matter 47 (1993) 8341.
V. R. Pradhan, J. W. Tierney, I. Wender and G. P. Huffman, Fuels 5 (1991) 497.
T. Osaki, H. Taoda, T. Horiuchi and H. Yamakita, React. Kinet. Catal. Lett. 51 (1993) 39.
A. Trovarelli, P. Matteazzi, G. Dolceti and A. Lutman, Appl. Catal. A Gen. 95 (1993) L9.
B. Breitscheidel, J. Zieder and U. Schubert, Chem. Mater. 3 (1991) 559.
C. M. Lukehart, J. P. Carpenter, S. B. Milne and K. J. Burnham, CHEMTECH August (1993) 29.
P. H. Tewari, A. J. Hunt and K. D. Lofftus, Mater. Lett. 3 (1985) 363.
JCPDS “Powder Diffraction File” (International Center for Diffraction Data, Swarthmore, PA, 1988).
C. J. Brinker and G. W. Scherer, “Sol-Gel Science” (Academic Press, San Diego, 1990).
A. W. Adamson, “Physical Chemistry of Surfaces”, 5th Edn (Wiley, New York, 1990)
H. Daamen, J. M. Ernsting and A. Oskam, Thermochim. Acta 33 (1979) 217.
A. G. Gilbert and K. G. P. Sulzmann, J. Electrochem. Soc. 121 (1993) 386.
R. Tenne, L. Margulis and G. Hodes, Adv. Mater. 5 (1993) 386.
R. Tenne, L. Margulis, M. Genut and G. Hodes, Nature 360 (1992) 444.
P. Fink, B. Muller and G. Rudakoff, J. Non-Cryst. Solids 145 (1992) 99.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ayers, M.R., Song, X.Y. & Hunt, A.J. Preparation of nanocomposite materials containing WS2, δ-WN, Fe3O4, or Fe9S10 in a silica aerogel host. JOURNAL OF MATERIALS SCIENCE 31, 6251–6257 (1996). https://doi.org/10.1007/BF00354446
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF00354446