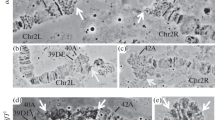
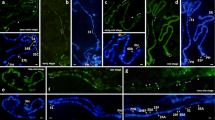
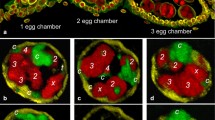
Abstract
Certain mutant alleles of the ovarian tumor (otu) locus give rise to polytene chromosomes in the pseudonurse cells (PNCs). We have previously shown that the banding pattern of these germ line-derived chromosomes is similar to that in the larval salivary gland chromosomes. In this study, we have examined the gene activity of these chromosomes. General gene expression from these chromosomes was studied by uridine autoradiography. The expression of specific genes was monitored by in situ hybridisation to mRNA and also by combining enhancer trap lines with otu mutants. We found that most of the genes studied were expressed in the PNCs as they were in the wild-type nurse cells. Four out of the 12 mRNAs studied accumulated in the nuclei instead of migrating to the cytoplasm. The intensity of accumulation directly correlated with the extent of polytenisation in the PNC nuclei. We suggest that the otu mRNA remains partly attached to the polytene chromosome template after transcription and discuss the effects of this phenomenon on polytenisation of the PNC chromosomes.
Similar content being viewed by others
References
Ashburner M (1970) Function and structure of polytene chromosomes during insect development. Adv Insect Physiol 7:1–95
Bellen HJ, O'Kane CJ, Wilson C, Grossniklaus U, Pearson RK, Gehring WJ (1989) P-element-mediated enhancer detection: a versatile method to study development in Drosophila. Genes Dev 3:1288–1300
Bertleth T, Burri M, Thoma G, Bopp D, Richstein S, Frigerio G, Noll M, Nüsslein-Volhard C (1988) The role of localisation of bicoid RNA in organising the anterior pattern of the Drosophila ovary. EMBO J 7:1749–1756
Bishop DL, King RC (1984) An ultrastructural study of ovarian development in the otu7 mutant of Drosophila melanogaster. J Cell Sci 67:87–119
Bohrmann J, Biber K (1994) Cytoskeleton-dependent transport of cytoplasmic particles in previtellogenic to mid-vitellogenic ovarian follicles of Drosophila: time-lapse analysis using video-enhanced microscopy. J Cell Sci 107:849–858
Bopp D, Horabin JI, Lersch RA, Cline TW, Schedl P (1993) Expression of the Sex-lethal gene is controlled at multiple levels during Drosophila oogenesis. Development 118:797–812
Boyd L, O'Toole E, Thummel CS (1991) Patterns E74 RNA and protein expression at the onset of metamorphosis in Drosophila. Development 112:981–995
Chandley AC (1987) Meiotic analysis in germ cells of man and the mouse. In: Monk M (ed) Mammalian development — a practical approach. IRL Press, Oxford, pp 71–91
Cooley L, Theurkauf WE (1994) Cytoskeletal functions during Drosophila oogenesis. Science 266:590–595
Corces V, Holmgren R, Freund R, Morimoto R, Meselson M (1980) Four heat shock proteins of Drosophila melanogaster coded within 12-kilobase region in chromosome subdivision 67B. Proc Natl Acad Sci USA 77:5390–5393
Ephrussi A, Dickinson LK, Lehmann R (1991) oskar organises the germ plasma and directs localisation of the posterior determinant nanos. Cell 66:37–50
Forlani S, Ferrandon D, Saget O, Mohier E (1993) A regulatory function for K10 in the establishment of dorsoventral polarity in the Drosophila egg and embryo. Mech Dev 41:109–120
Fuse N, Hirose S, Hayashi S (1994) Diploidy of Drosophila imaginal cells is maintained by a transcriptional repressor encoded by escargot. Genes Dev 8:2270–2281
Garcia-Bellido A, Robbins LG (1983) Viability of female germ line cells homozygous for zygotic lethals in Drosophila melanogaster. Genetics 103:235–247
Haenlin M, Roos C, Cassab A, Mohier E (1987) Oocyte-specific transcription of fs(1)K10: a Drosophila gene affecting dorsalventral polarity. EMBO J 6:801–807
Hammond MP, Laird CP (1985) Chromosome structure and DNA replication in nurse and follicle cells of Drosophila melanogaster. Chromosoma 91:267–278
Hashimoto C, Hudson KL, Anderson KV (1988) The Toll gene of Drosophila, required for dorsal-ventral embryonic polarity, appears to encode a transmembrane protein. Cell 52:269–279
Heino TI (1989) Polytene chromosomes from ovarian pseudonurse cells of the Drosophila melanogaster otu mutant. I. Photographic map of chromosome 3. Chromosoma 91:267–278
Heino TI (1994) Polytene chromosomes from ovarian pseudonurse cells of the Drosophila melanogaster otu mutant. II. Photographic map of the X chromosome. Chromosoma 103:4–15
Heitz JG, Plaut W (1975) Alteration of Ringer pH and its effects on precursor incorporation into Drosophila salivary gland nuclei. Chromosoma 51:147–155
Kalfayan L, Wensink PC (1981) α-tubulin genes of Drosophila. Cell 24:97–106
Kim-Ha J, Smith JL, Macdonald PM (1991) oskar messenger RNA is localised to the posterior pole of the Drosophila oocyte. Cell 66:23–36
King RC (1970) Ovarian development in Drosophila melanogaster. Academic Press, New York
King RC, Büning J (1985) The origin and functioning of insect oocytes and nurse cells. In: Kerkut GA, Gilbert LI (eds) Comprehensive insect physiology, biochemistry and pharmacology, vol 1. Pergamon Press, Oxford, pp 37–82
King RC, Bahns M, Horowitz R, Larramendi P (1978) A mutation that affects female and male germ cells differentially in Drosophila melanogaster Meigen (Diptera: Drosophilidae). Int J Insect Morphol Embryol 7:359–378
King RC, Riley SF, Cassidy JD, White PE, Paik YK (1981) Giant polytene chromosomes from the ovaries of a Drosophila melanogaster mutant. Science 212:441–443
King RC, Mohler DJ, Riley SF, Storto PD, Nicolazzo PS (1986) Complementation between alleles at the ovarian tumor (otu) locus of Drosophila melanogaster. Dev Genet 7:1–20
Kolmer M, Roos C, Tirronen M, Myöhänen S, Alho H (1994) Tissue-specific expression of the diazepam-binding inhibitor in Drosophila melanogaster: cloning, structure and localization of the gene. Mol Cell Biol 14:6983–6995
Kramer J, Zachar Z, Bingham PM (1994) Nuclear pre-mRNA metabolism: channels and tracks. Trends Cell Biol 4:35–37
Lehmann R, Nüsslein-Volhard C (1987) Involvement of the pumilio gene in the transport of an abdominal signal in the Drosophila embryo. Nature 329:167–170
Macdonald PM (1992) The Drosophila pumilio gene: an unusually long transcription unit and an unusual protein. Development 114:221–232
Mével-Ninio M, Terracol R, Kafatos FC (1991) The ovo gene of Drosophila encodes a zinc finger protein required for female germ line development. EMBO J 8:1559–1565
Mulligan PK, Rasch EM (1985) Determination of DNA content in the nurse and follicle cells from wild type and mutant Drosophila melanogaster by DNA-Feulgen cytophotometry. Histochemistry 82:233–247
Mulligan PK, Mohler JD, Kalfayan LJ (1988) Molecular localization and developmental expression of the otu locus of Drosophila melanogaster. Mol Cell Biol 8:1481–1488
Oliver B, Pauli D, Mahowald AP (1990) Genetic evidence that the ovo locus is involved in Drosophila germ line sex determination. Genetics 125:535–550
Oliver B, Kim Y-J, Baker BS (1993) Sex-lethal, a master and slave: a hierarchy of germ-line sex determination in Drosophila. Development 119:897–908
Oliver B, Singer J, Laget M, Pennetta G, Pauli D (1994) Function of Drosophila ovo+ in germ line sex determination depends on X chromosome number. Development 120:3185–3195
Painter TS, Reindorp EC (1939) Endomitosis in the nurse cells of the ovary of Drosophila melanogaster. Chromosoma 1:276–283
Pauli D, Oliver B, Mahowald AP (1993) The role of ovarian tumor locus in Drosophila melanogaster germ line sex determination. Development 119:123–134
Pokrywka N, Stephenson EC (1991) Microtubules mediate the localization of bicoid mRNA during Drosophila oogenesis. Development 113:55–66
Shermoen AW, O'Farrell PH (1991) Progression of the cell cycle through mitosis leads to abortion of nascent transcripts. Cell 67:303–310
Simon JA, Sutton CA, Lobell RB, Galser RL, Lis JT (1985) Determinants of heat shock-induced chromosome puffing. Cell 40:805–817
Sinha P, Mishra A, Lakhotia SC (1987) Chromosomal organization of Drosophila tumours. I. Polytene chromosome organisation and DNA synthesis in ovarian pseudonurse cells in otu mutants of D. melanogaster. Chromosoma 95:108–116
Smith AV, Orr-Weaver TL (1991) The regulation of cell cycle during Drosophila embryogenesis: The transition to polyteny. Development 112:997–1008
Sorsa V (1988) Chromosome maps of Drosophila, I and II. CRC Press, Boca Raton, Florida
Sprenger F, Nüsslein-Volhard C (1992) Torso receptor activity is regulated by a diffusible ligand produced at the extracellular terminal regions of the Drosophila egg. Cell 71:987–1001
Steinhauer WR, Kalfayan LJ (1992) A specific ovarian tumor protein isoform is required for efficient differentiation of germ cells in Drosophila oogenesis. Genes Dev 6:233–243
StJohnston D, Driever W, Berleth T, Richstein S, Nüsslein-Volhard C (1989) Multiple steps in the localization of bicoid RNA to the anterior pole of the Drosophila oocyte. Development 107 Suppl: 13–19
StJohnston D, Beuchle D, Nüsslein-Volhard C (1991) staufen: a gene required to localise maternal RNAs in the Drosophila egg. Cell 66:51–64
Storto PD, King RC (1988) Multiplicity of functions for the otu gene products during Drosophila oogenesis. Dev Genet 9:91–120
Suter B, Steward R (1991) Requirement for phosphorylation and localization of the Bicaudal-D protein in Drosophila oocyte. Cell 67:917–926
Suter B, Romberg LM, Steward R (1989) Bicaudal-D, a Drosophila gene involved in developmental asymmetry: localised transcript accumulation in ovaries and sequence similarity to myosin heavy chain tail domains. Genes Dev 3:1957–1968
Theurkauf WE, Baum H, Bo J, Wensink PC (1986) Tissue-specific and constitutive α-tubulin genes of Drosophila melanogaster code for structurally distinct proteins. Proc Natl Acad Sci USA 83:8477–8481
Theurkauf WE, Smiley S, Wong ML, Alberts BM (1992) Reorganization of the cytoskeleton during Drosophila oogenesis: implications for axis specification and intercellular transport. Development 115:923–936
Theurkauf WE, Alberts BM, Jan YN, Jongens TA (1993) A central role for microtubules in the differentiation of Drosophila oocytes. Development 118:1169–1180
Tirronen M, Partanen M, Heino TO, Heino TI, Roos C (1992) Analyses of the Drosophila quit, ovarian tumor and shut down mutants in oocyte differentiation using in situ hybridisation. Mech Dev 40:113–126
Tirronen M, Lahti V-P, Heino TI, Roos C (1995) Two otu transcripts are selectively localised in Drosophila oogenesis by a mechanism that requires a function of the otu protein. Mech Dev 52:65–75
Zachar Z, Kramer I, Mims P, Bingham PM (1993) Evidence for channelled diffusion of pre-mRNAs during nuclear RNA transport in metazoans. J Cell Biol 121:729–742
Zimmermann JL, Petri W, Meselson M (1983) Accumulation of a specific subset of D. melanogaster heat shock mRNAs in normal development without a heat shock. Cell 32:1161–1170
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Heino, T.I., Lahti, VP., Tirronen, M. et al. Polytene chromosomes show normal gene activity but some mRNAs are abnormally accumulated in the pseudonurse cell nuclei of Drosophila melanogaster otu mutants. Chromosoma 104, 44–55 (1995). https://doi.org/10.1007/BF00352225
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00352225