Abstract
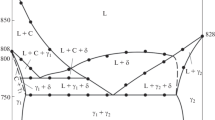
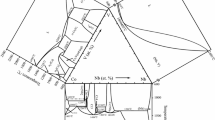
Before investigating the ternary system Bi2O3-CaO-CuO, a revision of the binary bounding system Bi2O3-CaO was first necessary. In the range between 20 and 70 mol % CaO the solid solutions β and α′'1 and the stoichiometric compounds Bi2CaO4, Bi6Ca4O13, and Bi2Ca2O5 were found to exist for 675 °C≦T≦780 °C. Above 780 °C a new high temperature compound with the formula Bi6Ca5O14 has been identified. The X-ray powder diffraction data, unit cell dimensions, as well as the space group, have been reported. The ternary system contains no intermediate compounds and also no solubility was found for the binary bounding phases. Below 780 °C all the Bi-Ca oxides mentioned above are in equilibrium with CuO. At 820 °C, a wide liquidus field is dominating so that these quasibinary equilibria disappear. For 780 °C<T<820°C the new compound Bi6Ca5O14 forms an equilibrium with Ca2CuO3 and CuO. Other equilibria are deduced. For a fixed ternary composition the reaction path of the sintering process was investigated as a function of time and temperature.
Similar content being viewed by others
References
K. Schulze, P. Majewski, B. Hettich and G. Petzow, Z. Metallkde 81 (1990) 836.
C.-L. Lee, J.-J. Chen, W.-J. Wen, T.-P. Perng, J.-M. Wu, T.-B. Wu and T.-S. Chin, J. Mater. Res. 5 (1990) 1403.
R. O. Suzuki, S. Kambara, H. Tsuchida, K. Shimizu and K. Ono, in “2nd International Symposium on Superconductivity” (ISS '89), (Springer-Verlag, Tokyo, 1990) Japan.
M. R. DeGuire, N. P. Bansal, D. E. Farrell, V. Finan, C. J. Kim, B. J. Hills and C.J. Allen, Phys. C (1990).
R. S. Roth, C. J. Rawn, B. P. Burton and F. Beech, J. Res. Nat. Inst. Stand. Technol 95 (1990) 291.
Y. Ikeda, H. Ito, S. Shimomura, Y. Oue, K. Inaba, Z. Hiroi and M. Takano, phys. C 159 (1989) 93.
R. S. Roth, C. J. Rawn, J. J. Ritter and B. P. Burton, J. Amer. Ceram. Soc. 72 (1989) 1545.
J. -C. Boivin, D. Thomas and G. Tridot, C.R. Acad. Sci. Paris C276 (1973) 1105.
M. Hovart and D. Kolar, J. Mater. Sci. Lett. 3 (1984) 659.
A. M. M. Gadalla and J. White, Trans. Brit. Ceram. Soc. 65 (1966) 181.
P. Conflant, J.-C. Boivin and D. Thomas, J. Solid State Chem. 18 (1976) 133.
E. M. Levin and R. S. Roth, J. Res. NBS 68A (1964) 197.
B. Aurivillius Arkiv. Kemi Mineral. Geol. 16A (17) (1943) 1.
J. B. Parise, C.C. Torardi, M.-H. Whangbo, C. J. Rawn, R. S. Roth and B.P. Burton, Chem. Mater 2 (1990) 455.
U.-C. Boshnke, I. Brunner and G. Zahn, to be published.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Boehnke, U.C., Heitman, P., Krötzsch, M. et al. Some details of the ternary system Bi2O3-CaO-CuO. JOURNAL OF MATERIALS SCIENCE 28, 111–116 (1993). https://doi.org/10.1007/BF00349040
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00349040