Abstract
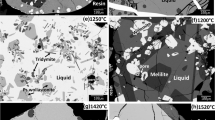
Phase equilibria of the binary PbO-CaO and ternary PbO-CaO-SiO2 systems have been investigated at 870-1655 °C for oxide liquid in equilibrium with air and solid oxide phases: tridymite or cristobalite SiO2, pseudowollastonite CaSiO3, dicalcium silicate (Ca,Pb)2SiO4, tricalcium silicate (Ca1−xPbx)3SiO5 (x < 0.03), new phase tricalcium-lead silicate (Ca0.8Pb0.2)3SiO5 (Ca12Pb3Si5O25), lime CaO, and calcium plumbate Ca2PbO4, covering the ranges of concentrations not studied before. High-temperature equilibration on primary phase or inert metal (platinum, gold) substrates, followed by quenching and direct measurement of the Pb, Ca and Si concentrations in the phases with the electron probe x-ray microanalysis (EPMA) has been used to accurately characterize the system. Liquidus phase equilibrium data of the present authors for the PbO-CaO-SiO2 system are essential to obtain a self-consistent set of parameters of thermodynamic models for all phases.




Similar content being viewed by others
References
M. Shevchenko and E. Jak, Experimental Liquidus Studies of the Pb-Fe-Si-O System in Equilibrium with Metallic Pb, Metall. Mater. Trans. B, 2017, 49(1), p 159-180
U. Kuxmann and P. Fischer, Beitrag zur Kenntnis der Zustandsdiagramme PbO-Al2O3, PbO-CaO, PbO-SiO2, Erzmetall (Z. Erzbergbau Metallhuettenwes), 1974, 27(11), p 533-537
H. Kitaguchi, J. Takada, K. Oda, and Y. Miura, Equilibrium Phase Diagram for the System PbO-CaO-CuO, J. Mater. Res., 1990, 5(5), p 929-931
D. Araten, Some Molten Ionic Oxides as Chemical Reagents, J. Appl. Chem. (London), 1968, 18(4), p 118-121
M. Koishi, A Study of the Hydration of Calcium Orthoplumbate.VII. The Mechanism of the Formation of the Hydrate, Bull. Chem. Soc. Jpn, 1966, 39(11), p 2406-2410
V.M. Tromel, Die kristallstruktur der verbindungen vom Sr2PbO4-typ, Z. Anorg. Allg. Chem., 1969, 371, p 237-247
E. Jak, N. Liu, and P.C. Hayes, Experimental study of Phase Equilibria in the Systems PbOx-CaO and PbOx-CaO-SiO2, Metall. Mater. Trans. B, 1998, 29B(3), p 541-553
E. Jak, S.A. Decterov, P.C. Hayes, and A.D. Pelton, Thermodynamic Optimisation of the Systems CaO-Pb-O and PbO-CaO-SiO2, Can. Metall. Q., 1998, 37(1), p 41-47
K.T. Jacob and K.P. Jayadevan, Measurement of Gibbs Energy of Formation of Ca2PbO4 Using a Solid-State Cel with Three Electrodes, J. Mater. Chem., 1997, 7(12), p 2407-2413
R.O. Suzuki, J.-I. Nagata, and D. Risold, Experimental Phase Equilibria in the PbOx-CaO System, J. Am. Ceram. Soc., 1998, 81(9), p 2493-2496
S.C. Samanta and F.A. Hummel, Phase Equilibria in the System CaO-PbO-SiO2, J. Am. Ceram. Soc., 1976, 59(3–4), p 157-160
G.A. Mikirticheva, R.G. Grebenshchikov, and S.K. Kuchaeva, Calcium Silicate (CaSiO3)-Lead Silicate (PbSiO3) Phase System, Zh. Neorg. Khim., 1989, 34(8), p 2135-2139 (in Russian)
M. Shevchenko, J. Chen, E. Jak, Establishing additional correction for quantitative EPMA measurements in the system PbO-SiO2, in AMAS 2017, 14th Biennial Australian Microbeam Analysis Symposium, 6-10 February, 2017 (Brisbane, Australia), QUT, Brisbane, pp 94–95
M. Shevchenko and E. Jak, Experimental Phase Equilibria Studies of the PbO-SiO2 System, J. Am. Ceram. Soc., 2018, 101(1), p 458-471
T. Hidayat, H.M. Henao, P.C. Hayes, and E. Jak, Phase Equilibria Studies of the Cu-Fe-O-Si System in Equilibrium with Air and with Metallic Copper, Metall. Mater. Trans. B, 2012, 43, p 1034-1045
E. Jak, P.C. Hayes, and H.-G. Lee, Improved Methodologies for the Determination of High Temperature Phase Equilibria, Korean J. Miner. Mater. Inst. (Seoul), 1995, 1(1), p 1-8
E. Jak, Integrated experimental and thermodynamic modelling research methodology for metallurgical slags with examples in the copper production field, in 9th International Conference on Molten Slags, Fluxes and Salts (MOLTEN12), 2012 (Beijing, China), The Chinese Society for Metals, W077
M. Shevchenko and E. Jak, Thermodynamic optimization of the binary PbO-CaO and ternary PbO-CaO-SiO 2 systems, Personal communication, The University of Queensland, Brisbane, 2018
Acknowledgments
The authors would like to thank Nyrstar (Australia), Outotec Pty Ltd (Australia), Aurubis AG (Germany), Umicore NV (Belgium), and Kazzinc Ltd, Glencore (Kazakhstan), and Australian Research Council Linkage project LP150100783 for their financial support for this research. The authors are grateful to Prof. Peter C. Hayes (UQ) for valuable comments and suggestions, to Ms. Suping Huang (UQ) for assistance with conducting experiments, and to the staff of the University of Queensland Centre for Microanalysis and Microscopy (CMM) for their support in maintenance and operation of scanning and electron microprobe facilities in the Centre.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shevchenko, M., Jak, E. Experimental Liquidus Study of the Binary PbO-CaO and Ternary PbO-CaO-SiO2 Systems. J. Phase Equilib. Diffus. 40, 148–155 (2019). https://doi.org/10.1007/s11669-019-00705-3
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11669-019-00705-3