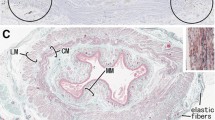
Abstract
The architecture and neurochemistry of the enteric nervous system was studied by use of whole-mount preparations obtained by microdissection of the horse jejunum. A myenteric plexus and two plexuses within the submucosa were identified. The external submucosal plexus lying in the outermost region of the submucosa had both neural and vascular connections with the inner submucosal plexus situated closer to the mucosa. Counts of neurones stained for NADH-diaphorase demonstrated the wide variation in size, shape and neurone content of individual ganglia in both the external and internal submucosal plexuses. The average number of cells/ganglion was similar in each plexus (about 25 cells). Immunoreactivities for galanin, vasoactive intestinal peptide and neuropeptide Y were observed in nerve cell bodies and fibres of each of the plexuses. Immunoreactivity for substance P was extensive and strong in nerve fibres of all plexuses but was weaker in cell bodies of the submucosal neurones and absent in the cell bodies of the myenteric plexus. Comparative quantitative analysis of immunoreactive cell populations with total cell numbers (enzyme staining) was indicative of neuropeptide colocalization in the external submucosal plexus.
Similar content being viewed by others
References
Bishop AE, Hodson NP, Major JH, Probert L, Yeats J, Edwards GB, Wright JA, Bloom SR, Polak JM (1984) The regulatory peptide system of the large bowel in equine grass sickness. Experientia 40:801–806
Bornstein JC, Costa M, Furness JB, Lees GM (1984) Electrophysiology and enkephalin immunoreactivity of identified myenteric plexus neurones of the guinea-pig small intestine. J Physiol 351:313–325
Burns GA, Cummings JF (1991) Equine myenteric plexus with special reference to the pelvic flexure pacemaker. Anat Rec 230:417–424
Burns GA, Cummings JF (1993) Neuropeptide distributions in the colon, caecum, and jejunum of the horse. Anat Rec 236:341–350
Burns GA, Karcher LF, Cummings JF (1990) Equine myenteric ganglionitis: a case of chronic intestinal pseudo-obstruction. Cornell Vet 80:53–63
Cooke HJ (1987) Neural and humoral regulation small intestinal electrolyte transport. In: Johnson LR (ed) Physiology of the gastrointestinal tract, 2nd edn, Raven Press, New York, pp 1307–1350
Costa M, Furness JB (1973) The simultaneous demonstration of adrenergic fibres and enteric ganglion cells. Histo J 5:343–349
Cummings JF, Sellers AF, Lowe JE (1984) Distribution of substance P-like immunoreactivity in the enteric neurones of the large colon of normal and amitraz-treated ponies: an immunocytochemical study. Equine Vet J 17:23–29
Dogiel AS (1899) Ueber den Bau der Ganglien in den Geflechten des Darmes und der Gallenblase des Menschen und der Säugetiere. Arch Anat Physiol Abt 1899:130–158
Furness JB, Costa M (1987) The enteric nervous system. Churchill Livingston, Edinburgh
Furness JB, Costa M, Keast JR (1984) Choline acetyltransferase-and peptide immunoreactivity of submucous neurones in the small intestine of the guinea-pig. Cell Tissue Res 237:329–336
Furness JB, Bornstein JC, Trussell DC (1988a) Shapes of nerve cells in the myenteric plexus of the guinea-pig small intestine revealed by the intracellular injection of dye. Cell Tissue Res 254:561–571
Furness JB, Llewellyn-Smith IJ, Bornstein JC, Costa M (1988b) Chemical neuroanatomy and the analysis of neuronal circuitry in the enteric nervous system. In: Björklund A, Hökfelt T, Owman C (eds) Handbook of chemical neuroanatomy, vol 6, the peripheral nervous system. Elsevier, Amsterdam, pp 161–218
Gabella G (1969) Detection of nerve cells by a histological technique. Experientia 25:218–219
Gabella G (1987) The number of neurones in the small intestine of mice, guinea-pigs and sheep. Neuroscience 22:737–752
Gunn M (1968) Histological and histochemical observations on the myenteric and submucous plexuses of mammals. J Anat 102:223–239
Hill CJ (1927) A contribution to our knowledge of the enteric plexuses. Phil Trans R Soc Lond [Biol] 215:355–387
Hirst GDS, McKirdy HC (1975) Synaptic potentials recorded from neurones in the submucous plexus of the guinea-pig small intestine. J Physiol [Lond] 249:369–385
Hoyle CHV, Burnstock G (1989a) Neuronal populations in the submucous plexus of the human colon. J Anat 166:7–22
Hoyle CHV, Burnstock G (1989b) Galanin-like immunoreactivity in enteric neurones of the human colon. J Anat 166:23–33
Hultgren BD (1982) Ileocolonic aganglionosis in white progeny of overo spotted horses. J Am Vet Med Assoc 180:289–292
Irwin DA (1931) The anatomy of Auerbach's plexus. Am J Anat 49:141–166
Katayama Y, Lees GM, Pearson GT (1986a) Electrophysiology and morphology of vasoactive-intestinal-peptide-immunoreactive neurones of the guinea-pig ileum. J Physiol [Lond] 378:1–11
Katayama Y, Lees GM, Pearson GT (1986) Electrophysiological and morphological similarities between myenteric plexus neurones showing immunoreactivity to enkephalins and to vasoactive intestinal peptide in guinea-pig isolated ileum. J Physiol [Lond] 378:101
Lees GM, Mackenzie GM, Pearson GT (1992) Complex correlations between the morphology, electrophysiology and peptide immunohistochemistry of guinea-pig enteric neurones. Eur J Morphol 30:1–14
Mannl A, Pospischil A, Dahme E (1986) Der Plexus submucosus (Meissner und Schabadasch) im Darm des Schweines. I. Licht-und elektronenmikroskopische Untersuchung der Normalstruktur. J Vet Med A 33:647–659
Melander T, Hökfelt T, Rokaeus A, Fahrenkrug Y, Tatemoto T, Mutt V (1985) Distribution of galanin-like immunoreactivity in the gastrointestinal tract of several mammalian species. Cell Tissue Res 239:253–270
Nishi S, North RA (1973) Intracellular recording from the myenteric plexus of the guinea-pig. J Physiol [Lond] 231:471–491
Obel A-L (1955) Studies on grass disease. The morphological picture with special reference to the vegetative nervous system. J Comp Pathol 65:334–346
Pearson GT, Woodman MP (1992) Neuropeptides and structural organization in the equine enteric nervous system. Dig Dis Sci 37:971
Pogson DM, Doxey DL, Gilmour JS, Milne EM, Chisholm HK (1992) Autonomic neurone degeneration in equine dysautonomia (grass sickness). J Comp Pathol 107:271–283
Scheuermann DW, Stach W (1984) Fluorescence microscopic study of the architecture and structure of an adrenergic network in the plexus myentericus (Auerbach), plexus submucosus externus (Schabadasch) and plexus submucosus internus (Meissner) of the porcine small intestine. Acta Anat 119:49–59
Scheuermann DW, Stach W, De Groodt-Lasseel MHA, Timmermans J-P (1987) Calcitonin gene-related peptide in morphologically well-defined Type II neurones of the enteric nervous system in the porcine small intestine. Acta Anat 129:325–328
Scheuermann DW, Stach W, Timmermans J-P, Adriaensen D, De Groodt-Lasseel MHA (1989) Neuron-specific enolase and S-100 protein immunohistochemistry for defining the structure and topographical relationship of the different enteric nerve plexuses in the small intestine of the pig. Cell Tissue Res 256:65–75
Schultzberg M, Hökfelt T, Nilsson G, Terenius L, Rehfeld JF, Brown M, Elde R, Goldstein M, Said S (1980) Distribution of peptide-and catecholamine-containing neurones in the gastrointestinal tract of rat and guinea-pig: immunohistochemical studies with antisera to substance P, vasoactive intestinal polypeptide, enkephalins, somatostatin, gastrin/cholecystokinin, neurotensin and dopamine β-hydroxylase. Neuroscience 5:689–744
Stach WA (1989) A revised morphological classification of neurones in the enteric nervous system. In: Singer MV, Goebell H (eds) Nerves and the gastrointestinal tract. Proceedings of the 50th Falk Symposium. MTP Press, Lancaster UK, pp 29–45
Timmermans J-P, Scheuermann DW, Stach W, Adriaensen D, De Groodt-Lasseel MHA (1990) Distinct distribution of CGRP-, enkephalin-, galanin-, neuromedin U-, neuropeptide Y-, somatostatin-, substance P-, VIP-and serotonin-containing neurons in the two submucosal ganglionic neural networks of the porcine small intestine. Cell Tissue Res 260:367–379
Timmermans J-P, Adriaensen D, Scheuermann DW, Stach W (1991) Morphological features of the enteric nervous system of an omnivorous animal, the domestic pig. J Auton Nerv Syst 33:193–194
Timmermans J-P, Scheuermann DW, Stach W, Adriaensen D, De Groodt-Lasscel MHA (1992a) Functional morphology of the enteric nervous system with special reference to large mammals. Eur J Morphol 30:113–122
Timmermans J-P, Scheuermann DW, Barbiers M, Adriaensen D, Stach W, Van Hee R, De Groodt-Lasseel MHA (1992b) Calcitonin gene-related peptide-like immunoreactivity in the human small intestine. Acta Anat 143:48–53
Timmermans J-P, Barbiers M, Scheuermann DW, Stach W, Adriaensen D, De Groodt-Lasseel MHA (1993) Occurrence, distribution and neurochemical features of small intestinal neurones projecting to the cranial mesenteric ganglion in the pig. Cell Tissue Res 272:49–58
Wilson AJ, Furness JB, Costa M (1981) The fine structure of the submucous plexus of the guinea-pig ileum. I. The ganglia, neurons, Schwann cells and neuropil. J Neurocytol 10:759–784
Wood JD (1987) Physiology of the enteric nervous system. In: Johnson LR (ed) Physiology of the gastrointestinal tract. 2nd edn. Raven Press, New York, pp 67–109
Young HM, Furness JB, Sewell P, Burcher EF, Kandiah CJ (1993) Total numbers of neurons in myenteric ganglia of the guinea-pig small intestine. Cell Tissue Res 272:197–200
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Pearson, G.T. Structural organization and neuropeptide distributions in the equine enteric nervous system: an immunohistochemical study using whole-mount preparations from the small intestine. Cell Tissue Res 276, 523–534 (1994). https://doi.org/10.1007/BF00343949
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00343949