Abstract
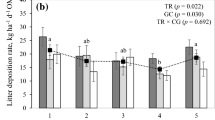
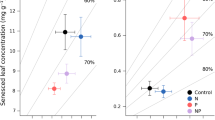
We investigated the effect of increased N-supply on productivity and potential litter decay rates of Carex species, which are the dominant vascular plant species in peatlands in the Netherlands. We hypothesized that: (1) under conditions of N-limited plant growth, increased N-supply will lead to increased productivity but will not affect C:N ratios of plant litter and potential decay rates of that litter; and (2) under conditions of P-limited plant growth, increased N-supply will not affect productivity but it will lead to lower C:N ratios in plant litter and thereby to a higher potential decay rate of that litter. These hypotheses were tested by fertilization experiments (addition of 10 g N m-2 year-1) in peatlands in which plant growth was N-limited and P-limited, respectively. We investigated the effects of fertilization on net C-fixation by plant biomass, N uptake, leaf litter chemistry and potential leaf litter decay. In a P-limited peatland, dominated by Carex lasiocarpa, there was no significant increase of net C-fixation by plant biomass upon enhanced N-supply, although N-uptake had increased significantly compared with the unfertilized control. Due to the N-fertilization the C:N ratio in the plant biomass decreased significantly. Similarly, the C:N ratio of leaf litter produced at the end of the experiment showed a significant decrease upon enhanced N-supply. The potential decay rate of that litter, measured as CO2-evolution from the litter under aerobic conditions, was significantly increase upon enhanced N-supply. In a N-limited peatland, dominated by C. acutiformis, the net C-fixation by plant biomass increased with increasing N-supply, whereas the increase in N-uptake was not significant. The C:N ratio of both living plant material and of dead leaves did not change in response to N-fertilization. The potential decay rate of the leaf litter was not affected by N-supply. The results agree with our hypotheses. This implies that atmospheric N-deposition may affect the CO2-sink function of peatlands, but the effect is dependent on the nature of nutrient limitation. In peatlands where plant growth is N-limited, increased N-supply leads to an increase in the net accumulation of C. Under conditions of P-limited plant growth, however, the net C-accumulation will decrease, because productivity is not further increased, whereas the amount of C lost through decomposition of dead organic matter is increased. As plant growth in most terrestrial ecosystems is N-limited, increased N-supply will in most peatlands lead to an increase of net C-accumulation.
Similar content being viewed by others
References
Aerts R (1989) The effect of increased nutrient availability on leaf turnover and aboveground productivity of two evergreen ericaceous shrubs. Oecologia 78: 115–120
Aerts R (1993) Biomass and nutrient dynamics of dominant plant species in heathlands. In: Aerts R, Heil GW (eds) Heathlands, patterns and processes in a changing environment. Kluwer Academic Publishers, London, pp 51–84
Aerts R, Berendse F (1988) The effect of increased nutrient availability on vegetation dynamics in wet heathlands. Vegetatio 76: 63–69
Aerts R, De Caluwe H (1989) Aboveground productivity and nutrient turnover of Molinia caerulea along an experimental gradient of nutrient availability. Oikos 54: 320–324
Aerts R, Wallén B, Malmer N (1992a) Growth-limiting nutrients in Sphagnum-dominated bogs subject to low and high atmospheric nitrogen supply. J Ecol 80: 131–140
Aerts R, De Caluwe H, Konings H (1992b) Seasonal allocation of biomass and nitrogen in four Carex species from mesotrophic and eutrophic fens as affected by nitrogen supply. J Ecol 80: 653–664
Asman WAH, Drukker B, Janssen AJJ (1988) Modelled historical concentrations of depositions of ammonia and ammonium in Europe. Atmos Environ 22: 359–367
Berendse F (1990) Organic matter accumulation and nitrogen mineralization during secondary succession in heathland ecosystems. J Ecol 78: 413–427
Berg B, Staaf H (1980) Decomposition rate and chemical changes in decomposing needle litter of Scots pine. II. Influence of chemical composition. In: T Persson (ed) Structure and function of northern coniferous forest. Ecol Bull 32: 373–390
Bietz JA (1974) Micro-Kjeldahl analysis by an improved automated ammonia determination following manual digestion. Anal Chem 46: 1617–1618
Billings WD (1987) Carbon balance of Alaskan tundra and taiga ccosystems: past, present and future. Q Sci Rev 6: 165–177
Björk S (1967) Ecologic investigations of Phragmites communis. Fol Limn Scand 14: 1–248
Bobbink R, Heil GW (1993) Atmospheric deposition of sulphur and nitrogen in heathland ecosystems. In: Aerts R, Heil HW (eds) Heathlands, patterns and processes in a changing environment. Kluwer London, pp 25–50
Bobbink R, Bik L, Willems JH (1988) Effects of nitrogen fertilization on vegetation structure and dominance of Brachypodium pinnatum (L.) Beauv. in chalk grassland. Acta Bot Neerl 37: 231–242
Chapin FS, Jefferies RL, Reynolds JF, Shaver GR, Svoboda J (1992) Arctic ecosystems in a changing climate. An ecophysiological perspective. Academic Press, New York
Clymo RS (1984) The limits to peat bog growth. Philos Trans R Soc Lond B 303: 605–654
Coulson JC, Butterfield J (1978) An investigation of the biotic factors determining the rates of plant decomposition on blanket bog. J Ecol 66: 631–650
Fog K (1988) The effect of added nitrogen on the rate of decomposition of organic matter. Biol Rev 63: 433–462
French DD (1988) Some effects of changing soil chemistry on decomposition of plant litters and cellulose on a Scottish moor. Oecologia 75: 608–618
Fritschen LJ, LLoyd WG (1979) Environmental Instrumentation. Springer, Berlin Heidelberg New York
Gorham E (1991) Northern peatlands; role in the carbon cycle and probable responses to climatic warming. Ecol Appl 1: 182–195
Gorham E (1994) The future of research in Canadian peatlands: a brief survey with particular reference to global change. Wetlands 14: 206–215
Heÿ GJ, Schneider T (eds) (1991) Acidification Research in The Netherlands. Studies in Environmental Science 46. Elsevier, Amsterdam
Hogg EH (1993) Decay potential of hummock and hollow Sphagnum peats at different depths in a Swedish raised bog. Oikos 66: 269–278
Hunt HW, Ingham ER, Coleman DC, Elliot ET, Reid CPP (1988) Nitrogen limitation of production and decomposition in prairie mountain meadow, and pine forest. Ecology 69: 1009–1016
Jonasson S, Havström M, Jensen M, Callaghan TV (1993) In situ mineralization of nitrogen and phosphorus of arctic soils after perturbations simulating climate change. Oecologia 95: 179–196
Koerselman W, Verhoeven JTA (1992) Nutrient dynamics in mires of various trophic status: nutrient inputs and outputs and the internal nutrient cycle. In: JTA Verhoeven (ed) Fens and bogs in the Netherlands: vegetation, history, nutrient dynamics and conservation. Kluwer, London, pp 397–432
Kvet J, Husak S (1978) Primary data on biomass and production estimates in typical stands of fishpond littoral communities. In: Dykyjová D, Kvet J (eds) Pond littoral ecosystems, structure and Functions. (Ecological Studies no 28) Springer, Berlin Heidelberg New York, pp 211–220
Lennox LJ (1979) An automated procedure for the determination of phosphorus. Water Res 13: 1329–1333
Malmer N (1990) Constant or increasing nitrogen concentrations in Sphagnum mosses in mires in Southern Sweden during the last few decades. Aquilo Ser Bot 28: 57–65
Malmer N, Wallén B (1993) Accumulation and release of organic matter in ombrotrophic bog hummocks-processes and regional variation. Ecography 16: 193–211
Melillo JM, McGuire AD, Kicklighter DW, Moore B, Vorosmarty CJ, Schloss AL (1993) Global climate change and terrestrial net primary production. Nature 363: 234–240
Oechel WC, Hastings SJ, Vourlitis G, Jenkins M, Riechers G, Grulke N (1993) Recent change of Arctic tundra from a net carbon dioxide sink to a source. Nature 361: 520–523
Page AC, Miller RH, Keeney DR (1982) Methods of soil analyses. Part 2. Chemical and microbiological properties. American Society of Agronomy, Madison, Wisconsin
Pastor J, Stillwell MA, Tilman D (1987) Little bluestem litter dynamics in Minnesota old fields. Oecologia 72: 327–330
Ruuhijärvi R (1983) The Finnish mire types and their distribution. In: Gore AJP (ed) Ecosystems of the world, vol 4B. Mires: swamp, bog, fen, and moor. Elsevier, Amsterdam, pp 47–67
SAS Institute Inc (1985) SAS/STAT guide for personal computers, version 6 edn. SAS Institute Inc, Cary, NC
Shaver GR, Billings WD, Chapin FS, Giblin AE, Oechel WC, Nadelhoffer KJ, Rastetter EB (1992) Global change and the carbon balance of arctic ecosystems. Bioscience 42: 433–441
Tamm CO (1954) Some observations on the nutrient turn-over in a bog community dominated by Eriophorum vaginatum L. Oikos 5: 189–194
Taylor BR, Parkinson D (1988a) A new microcosm approach to litter decomposition studies. Can J Bot 66: 1933–1939
Taylor BR, Parkinson D (1988b) Respiration and mass loss rates of aspen and pine leaf litter decomposing in laboratory microcosms. Can J Bot 66: 1948–1959
Taylor BR, Parkinson D, Parsons WFJ (1989) Nitrogen and lignin content as predictors of litter decay rates: a microcosm test. Ecology 70: 97–104
Tian G, Kang BT, Brussaard L (1992) Effects of chemical composition on N, Ca, and Mg release during incubation of leaves from selected agroforestry and fallow plant species. Biogeochemistry 16: 103–119
Tilman D (1984) Plant dominance along an experimental nutrient gradient. Ecology 65: 1445–1453
Van Vuuren MMI, Van der Eerden LJ (1992) Effects of three rates of atmospheric nitrogen deposition enriched with 15N on litter decomposition in a heathland. Soil Biol Biochem 24: 527–532
Verhoeven JTA, Arts HHM (1987) Nutrient dynamics in small mesotrophic fens surrounded by cultivated land. II. N and P accumulation in plant biomass in relation to the release of inorganic N and P in the peat soil. Occologia 72: 557–561
Verhoeven JTA, Schmitz MB (1991) Control of plant growth by nitrogen and phosphorus in mesotrophic fens. Biogeochemistry 12: 135–148
Verhoeven JTA, Van Beek S, Dekker M, Storm W (1983) Nutrient dynamics in small mesotrophic fens surrounded by cultivated land. I. Productivity and nutrient uptake by the vegetation in relation to the flow of eutrophicated ground water. Oecologia 60: 25–33
Verhoeven JTA, Schmitz MB, Pons TL (1988) Comparative demographic study of Carex rostrata Stokes, C. diandra Schrank and C. acutiformis Ehrh. in fens of different nutrient status. Aquat Bot 30: 95–108
Verhoeven JTA, Maltby E, Schmitz MB (1990) Nitrogen and phosphorus mineralization in fens and bogs. J Ecol 78: 713–726
Vermeer JG (1986a) The effect of nutrients on shoot biomass and species composition of wetland and hayfield communities. Acta Oecol Oecol Plant 7: 31–41
Vermeer JG (1986b) The effect of nutrient addition and lowering of the water-table on shoot biomass and species composition of a wet grassland community (Cirsio-Molinietum Siss. et de Vries, 1942). Acta Oecol Oecol Plant 7: 145–155
Vitousek PM, Howarth RW (1991) Nitrogen limitation on land and in the sea: how can it occur? Biogeochemistry 13: 87–115
Wieder RK, Yavitt JB (1994) Peatlands and global climate change: insights from comparative studies of sites situated along a latitudinal gradient. Wetlands 14: 229–238
Witkamp M (1966) Decomposition of leaf litter in relation to environment, microflora, and microbial respiration. Ecology 47: 194–201
Woodwell GM (1994) Biotic feedbacks in the global climate system. Oxford University Press, Oxford
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Aerts, R., van Logtestijn, R., van Staalduinen, M. et al. Nitrogen supply effects on productivity and potential leaf litter decay of Carex species from peatlands differing in nutrient limitation. Oecologia 104, 447–453 (1995). https://doi.org/10.1007/BF00341342
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00341342