Summary
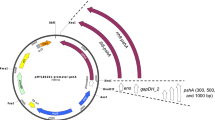
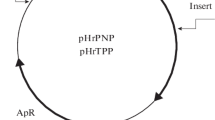
The structural genes (hup) of the H2 uptake hydrogenase of Rhodobacter capsulatus were isolated from a cosmid gene library of R. capsulatus DNA by hybridization with the structural genes of the H2 uptake hydrogenase of Bradyrhizobium japonicum. The R. capsulatus genes were localized on a 3.5 kb HindIII fragment. The fragment, cloned onto plasmid pAC76, restored hydrogenase activity and autotrophic growth of the R. capsulatus mutant JP91, deficient in hydrogenase activity (Hup-). The nucleotide sequence, determined by the dideoxy chain termination method, revealed the presence of two open reading frames. The gene encoding the large subunit of hydrogenase (hupL) was identified from the size of its protein product (68108 dalton) and by alignment with the NH2 amino acid protein sequence determined by Edman degradation. Upstream and separated from the large subunit by only three nucleotides was a gene encoding a 34 256 dalton polypeptide. Its amino acid sequence showed 80% identity with the small subunit of the hydrogenase of B. japonicum. The gene was identified as the structural gene of the small subunit of R. capsulatus hydrogenase (hupS). The R. capsulatus hydrogenase also showed homology, but to a lesser extent, with the hydrogenase of Desulfovibrio baculatus and D. gigas. In the R. capsulatus hydrogenase the Cys residues, (13 in the small subunit and 12 in the large subunit) were not arranged in the typical configuration found in [4Fe−4S] ferredoxins.
Similar content being viewed by others
References
Adman ET, Sieker LC, Jensen LH (1973) The structure of a bacterial ferredoxin. J Biol Chem 248:3987–3996
Birnboim HC, Doly J (1979) A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res 7:1513–1523
Boyer HW, Roulland-Dussoix D (1969) A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol 41:459–472
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Bruschi M, Guerlesquin F (1988) Structure, function and evolution of bacterial ferredoxins. FEMS Microbiol Rev 54:155–176
Cantrell MA, Haugland RA, Evans HJ (1983) Construction of a Rhizobium japonicum gene bank and use in isolation of a hydrogen uptake gene. Proc Natl Acad Sci USA 80:181–185
Colbeau A, Vignais PM (1981) The membrane-bound hydrogenase of Rhodopseudomonas capsulata. Stability and catalytic properties. Biochim Biophys Acta 662:271–284
Colbeau A, Vignais PM (1983) The membrane-bound hydrogenase of Rhodopseudomonas capsulata is inducible and contains nickel. Biochim Biophys Acta 748:128–138
Colbeau A, Chabert J, Vignais, PM (1983) Purification, molecular properties and localization in the membrane of the hydrogenase of Rhodopseudomonas capsulata. Biochim Biophys Acta 748:116–127
Colbeau A, Godfroy A, Vignais PM (1986) Cloning of DNA fragments carrying hydrogenase genes of Rhodopseudomonas capsulata. Biochimie 68:147–155
Ditta G, Stanfield S, Corbin D, Helinski DR (1980) Broad host range DNA cloning system for Gram-negative bacteria: construction of a gene bank of Rhizobium meliloti. Proc Natl Acad Sci USA 77:7347–7351
Friedman AM, Long SR, Brown SE Buikema WJ, Ausubel FM (1982) Construction of a broad host range cosmid cloning vector and its use in the genetic analysis of Rhizobium mutants. Gene 18:289–296
Friedrich B, Kortlüke C, Hogrefe C, Eberz G, Silber B, Warrelmann J (1986) Genetics of hydrogenase from aerobic lithoautotrophic bacteria. Biochimie 68:133–145
Gogotov IN (1986) Hydrogenases of phototrophic microorganisms. Biochimie 68:181–187
Hager DA, Burgess RR (1980) Elution of proteins from SDS polyacrylamide gels, removal of SDS and renaturation of enzymatic activity: results with subunit of E. coli RNA polymerase, wheat germ DNA topoisomerase and other enzymes. Anal Biochem 109:76–86
Hattori M, Sakaki Y (1986) Dideoxy sequencing method using denatured plasmid templates. Anal Biochem 152:232–238
Hillmer P, Gest H (1977) H2 metabolism in the photosynthetic bacterium Rhodopseudomonas capsulata: H2 production by growing cultures. J Bacteriol 129:720–731
Hohn B, Collins J (1980) A small cosmid, for efficient cloning of large DNA fragments. Gene 11:291–298
Imhoff JF, Trüper HG, Pfennig N (1984) Rearrangement of the species and genera of the phototrophic “purple non sulfur bacteria”. Int J Syst Bacteriol 34:340–343
Ish-Horowicz D, Burke JF (1981) Rapid and efficient cosmid, cloning. Nucleic Acids Res 9:2989–2998
Kortlücke C, Hogrefe C, Eberz G, Pühler A, Friedrich B (1987) Genes of lithoautotrophic metabolism are clustered on the megaplasmid pHG1 in Alcaligenes eutrophus. Mol Gen Genet 210:122–128
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Lambert GR, Cantrell MA, Hanus FJ, Russell SA, Haddad KR, Evans HJ (1985) Intra and interspecies transfer and expression of Rhizobium japonicum hydrogen uptake genes and autotrophic growth capability. Proc Natl Acad Sci USA 82:3232–3236
Leclerc M (1988) Génétique de l'hydrogénase chez Rhodobacter capsulatus: séquençage de gènes et identification des ARNm. Thesis, University of Grenoble, France
Levitt M (1976) A simplified representation of protein conformations for rapid simulation of protein folding. J Mol Biol 104:59–107
Li C, Peck HD Jr, LeGall J, Przybyla AE (1987) Cloning, characterization, and sequencing of the genes encoding the large and small subunits of the periplasmic (NiFe)hydrogenase of Desulfovibrio gigas. DNA 6:539–551
Maniatis T, Fritsch EF, Sambrook J (1982) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York
Marmur J (1961) A procedure for the isolation of deoxyribonucleic acid from micro-organisms. J Mol Biol 3:208–218
Marrs B (1974) Genetic recombination in Rhodopseudomonas capsulata. Proc Natl Acad Sci USA 71:971–973
Menon NK, Peck Jr HD, Le Gall J, Przybyla AE (1987) Cloning and sequencing of the genes encoding the large and small subunits of the periplasmic (NiFeSe) hydrogenase of Desulfovibrio baculatus. J Bacteriol 169:5401–5407
Mizusawa S, Nishumura S, Seela F (1986) Improvement of the dideoxy chain termination method of DNA sequencing by use of deoxy-7-deazaguanosine triphosphate in place of dGTP. Nucleic Acids Res 14:1319–1324
Morrison DA (1979) Transformation and preservation of competent bacterial cells by freezing. Methods Enzymol 68:326–331
Needleman SB, Wunsch CD (1970) A general method applicable to the search for similarities in the amino acid sequence of two proteins. J Mol Biol 48:443–452
Poncz M, Solowiejczyk D, Ballantine M, Schwartz E, Surrey S (1982) “Nonrandom” DNA sequence analysis in bacteriophage M13 by the dideoxy chain-termination method. Proc Natl Acad Sci USA 79:4298–4302
Prickril BC, He SH, Li C, Menon N, Choi ES, Przybyla AE, Dervartanian DV, Peck Jr HD, Fauque G, Le Gall J, Teixiera M, Moura I, Moura JJG, Patil D, Huynh BH (1987) Identification of three classes of hydrogenase in the genus Desulfovibrio. Biochem Biophys Res Commun 149:369–377
Sanger F, Nicklen S, Coulson AR (1977) DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA 74:5463–5467
Sankar P, Lee JH, Shanmugam KT (1985), Cloning of hydrogenase genes and fine structure analysis of an operon essential for H2 metabolism in Escherichia coli. J Bacteriol 162:353–360
Sayavedra L, Powell G, Morris R, Evans HJ (1988) Nucleotide sequence of the genetic loci encoding subunits of Bradyrhizobium japonicum uptake hydrogenase. 7th International Congress on N2 Fixation, Cologne, FRG, March (Abst. 12–44)
Seefeldt LC, MacCollum LC, Doyle CM, Arp DJ (1987) Immunological and molecular evidence for a membrane-bound dimeric hydrogenase in Rhodopseudomonas capsulata. Biochim Biophys Acta 914:299–303
Staden R, McLachlan AD (1982) Codon preference and its use in identifying protein coding regions in long DNA sequences. Nucleic Acids Res 10:141–174
Strauss EC, Kobori JA, Siu F, Hood LE (1986) Specific primer directed DNA sequencing. Anal Biochem 154:353–360
Tibelius KH, Robson RL, Yates MF (1987) Cloning and characterization of hydrogenase genes from Azotobacter chroococcum. Mol Gen Genet 206:285–290
Vignais PM, Colbeau A, Willison JC, Jouanneau Y (1985) Hydrogenase, nitrogenase and hydrogen metabolism in the photosynthetic bacteria. Adv Microbial Physiol 26:155–234
Voordouw G, Brenner S (1985) Nucleotide sequence of the gene encoding the hydrogenase from Desulfovibrio vulgaris (Hildenborough). Eur J Biochem 148:515–520
Weaver PF, Wall JD, Gest H (1975) Characterization of Rhodopseudomonas capsulata. Arch Microbiol 105:207–216
Willison JC, Magnin JP, Vignais PM (1987) Isolation and characterization of Rhodobacter capsulatus strains lacking endogenous plasmids. Arch Microbiol 147:134–143
Wu LF, Mandrand-Berthelot MA (1986) Genetic and physiological characterization of new Escherichia coli mutants impaired in hydrogenase activity. Biochimie 68:167–179
Yanisch-Perron C, Vieira J, Messing J (1985) Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13m18 and pUC19 vectors. Gene 33:103–119
Youvan DC, Alberti M, Begusch H, Bylina EJ, Hearst JE (1984) Reaction center and light-harvesting I genes from Rhodopseudomonas capsulata. Proc Natl Acad Sci USA 81:189–192
Yu PL, Hohn B, Falk H, Drews G (1982) Molecular cloning of the ribosomal RNA genes of the photosynthetic bacterium Rhodopseudomonas capsulata. Mol Gen Genet 188:392–398
Zuber M, Harker AR, Sultana MA, Evans HJ (1986) Cloning and expression of Bradyrhizobium japonicum uptake hydrogenase structural genes in Escherichia coli. Proc Natl Acad Sci USA 83:7668–7672
Author information
Authors and Affiliations
Additional information
Communicated by H. Hennecke
Rights and permissions
About this article
Cite this article
Leclerc, M., Colbeau, A., Cauvin, B. et al. Cloning and sequencing of the genes encoding the large and the small subunits of the H2 uptake hydrogenase (hup) of Rhodobacter capsulatus . Mol Gen Genet 214, 97–107 (1988). https://doi.org/10.1007/BF00340186
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00340186