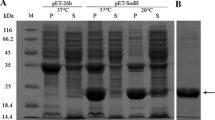
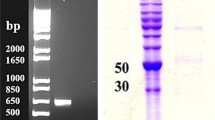
Summary
The E. coli iron superoxide dismutase gene (sodB) was utilized as a heterologous probe to isolate a superoxide dismutase (sod) gene from Anacystis nidulans R2. Nucleotide sequence analysis revealed a 603 bp open reading frame with deduced amino acid sequence similar to other sod genes and to cyanobacterial superoxide dismutase amino-terminal sequences. Assuming proteolytic cleavage of the initial methionine residue, the molecular mass of the mature A. nidulans R2 sodB polypeptide is 22000 daltons. Only a single copy of the superoxide dismutase sequence was detected in the A. nidulans R2 genome using Southern hybridization. Northern hybridization analysis indicated a single, monocistronic RNA transcript of approximately 720 bases. Primer extension mapping localized the transcription start site to 46 bases upstream from the initial methionine residue. A single orientation of a 2.1 kb PstI fragment containing the entire sod gene cloned into pUC18 was able to complement E. coli sodAsodB mutants. Complementation of the E. coli mutants was based on the ability of the cells to grow aerobically on minimal glucose medium. Growth curves of the complemented E. coli sodAsodB mutants showed that these cells exhibited levels of resistance to paraquat comparable to that of the wild-type E. coli phenotype.
Similar content being viewed by others
References
Abelovich A, Shilo M (1972) Photoxidative death in blue-green algae. J Bacteriol 11:682–689
Abelovich A, Kellenberg D, Shilo M (1974) Effect of photooxidative conditions on levels of superoxide dismutase in Anacystis nidulans. Photochem Photobiol 19:379–382
Allen M (1968) Simple conditions for growth of unicellular blue-green algae on plates. J Phycol 4:1–3
Asada K, Yoshikama K, Takahashi M, Maeda Y, Enmanji K (1975) Superoxide dismutase from a blue green alga, Plectonema boryanum J Biol Chem 250:2801–2807
Asada K, Kanematsu S, Vchida K (1977) Superoxide Dismutase in Photosynthetic Organisms: Absence of the Cuprozinec enzyme in eukaryotic algae. Arch Biochem Biophys 179:243–256
Barra D, Schinina ME, Bannister WH, Bannister JV, Bossa F (1987) The primary structure of iron-superoxide dismutase from Photobacterium leiognathi. J Biol Chem 262:1001–1009
Belasco JG, Beatty T, Adams CW, Gabain A von, Cohen SN (1985) Differential expression of photosynthesis genes in R. capsulata results from segmental differences in stability within the polycistronic rxcA transcript. Cell 40:171–181
Beyer WF Jr, Fridovich I (1987) Effect of hydrogen peroxide on the iron containing superoxide dismutase of Escherichia coli. Biochemistry 26:1251–1257
Bloch CA, Ausubel FM (1986) Paraquat-mediated selection for mutations in the manganese-superoxide dismutase gene sodA. J Bacteriol 168:795–798
Borbely G, Simoncsits A (1981) 3′-terminal conserved loops of 16S rRNAs from the cyanobacterium Synechococcus AN PCC 6301 and maize chloroplast differ only in two bases. Biochem Biophys Res Commun 101:846–852
Brock CJ, Harris JI (1977) Superoxide dismutase from Bacillus stearothermophilus—Reversible removal of manganese and its replacement by other metals. Biochem Soc Trans 5:1537–1539
Brock CJ, Walker JE (1980) Superoxide dismutase from Bacillus stearothermophilus—Complete amino acid sequence of a manganese enzyme. Biochemistry 19:2873–2882
Carlioz A, Touati D (1986) Isolation of superoxide dismutase mutants in Escherichia coli: is superoxide dismutase necessary for aerobic life. EMBO J 5:623–630
Carlioz A, Ludwig ML, Stalling WC, Free JA, Steiman HM, Touati D (1988) Iron superoxide dismutase: Nucleotide sequence of the gene from E. coli K12 and correlations with crystal structure. J Biol Chem 263:1555–1562
Fee JA, Shapiro EG, Moss TH (1976) Direct evidence for manganese (III) binding to superoxide dismutase of Escherichia coli B. J Biol Chem 251:6157–6159
Fridovich I (1975) Superoxide dismutase. Annu Rev Biochem 44:147–159
Golden SS, Brusslan J, Haselkorn R (1986) Expression of a family of psbA genes encoding a photosystem II polypeptide in the cyanobacterium Anacystis nidulans R2 EMBO J 5:2789–2798
Gregory EM, Fridovich I (1973) Oxygen toxicity and the superoxide dismutase. J Bacteriol 114:1193–1197
Halliwell B (1978) Chloroplasts at work-review of modern developments in our understanding of chloroplast metabolism. Progr Biophys Mol Biol 33:1–54
Harris J, Auffret AD, Northrop FD, Walker JE (1980) Structural comparisions of superoxide dismutases. Eur J Biochem 106:297–303
Klug G, Adams CW, Belasco J, Doerge B, Cohen SN (1987) Biological consequences of segmental alterations in mRNA stability: effects of deletion of the intercistronic hairpin loop region of the Rhodobacter capsulatus puf operon. EMBO J 6:3515–3520
Laudenbach DE, Reith ME, Straus NA (1988) Isolation, sequence analysis, and transcriptional studies of the flavodoxin gene from Anacystis nidulans R2. J Bacteriol 170:258–265
Lumsden J, Cammack R, Hall DO (1975) Superoxide dismutase in photosynthetic organisms provides an evolutionary hypothesis. Nature 257:670–672
Lumsden J, Cammack R, Hall DO (1976) Purification and physical properties of superoxide dismutase from two photosynthetic microorganisms. Biochim Biophys Acta 438:380–392
Maniatis TE, Fritsch EF, Sambrook J (1982) In: Molecular cloning. A laboratory manual, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York
Miller JH (1972) In: Experiments in Molecular Genetics, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York
Misra HP, Keele BB (1975) Purification properties of superoxide dismutase from a blue-green algae. Biochim Biophys Acta 379:418–425
Okada S, Kanematsu S, Osada K (1979) Intracellular distribution of manganese and ferric superoxide dismutases in blue-green algae. FEBS Letts 103:106–110
Reith ME, Laudenbach DE, Straus NA (1986) Isolation and nucleotide sequence analysis of the ferredoxin I gene from the cyanbacterium Anacystis nidulans R2. J Bacteriol 168:1319–1324
Ringe D, Petsko GA, Yamakura F, Suzuki K, Ohmori D (1983) Structure of iron superoxide dismutase from Pseudomonas ovalist at 2.9 Å resolution. Proc Natl Acad Sci USA 80:3879–3883
Rosenberg M, Court D (1979) Regulatory sequences involved in the promotion and termination of RNA transcription. Annu Rev Genet 13:319–353
Sakamoto H, Tonati D (1984) Cloning of the iron superoxide dismutase gene (sodB) in Escherichia coli K-12. J Bacteriol 159:418–420
Scott MD, Meshnick SR, Eaton JW (1987) Superoxide dismutaserich bacteria: Paradoxical increase in oxidant toxicity. J Biol Chem 262:3640–3645
Stallings WC, Powers TB, Pattridge KA, Fee JA, Ludwig ML (1983) Iron superoxide dismutase from E. coli at 3.1 Å resolution—A structure unlike that of copper-zinc proteins at both monomer and dimer levels. Proc Natl Acad Sci USA 80:3884–3888
Stallings WC, Pattridge KA, Strong RK, Ludwig ML (1984) Manganese and iron superoxide dismutases are structural homologs. J Biol Chem 259:10695–10699
Stallings WC, Pattridge KA, Strong RK, Ludwig ML (1985) The structure of manganese superoxide dismutase from thermusthermophilus HB 8 at 2.4 Å resolution. J Biol Chem 260:16424–16432
Steinitz Y, Mazor Z, Shilo M (1979) A mutant of the cyanobacterium Plectonema boryanum resistant to photooxidation. Plant Sci Letts:327–335
Steinman HM (1982) Copper-zinc superoxide dismutases from Caulobacter crescentus CB 15-Å novel bacteriocuprein form of the enzyme. J Biol Chem 257:10283–10292
Stern DE, Gruissem W (1987) Control of plastid gene expression: 3′ inverted repeats act as mRNA processing and stabilizing elements, but do not terminate transcription. Cell 51:1145–1157
Takeda Y, Avila H (1986) Structure and gene expression of the E. coli Mn-superoxide dismutase gene. Nucleic Acids Res 14:4577–4588
Tandeau de Marsac N, Borrias WE, Kuhlemeier CJ, Castetes AM, Arkel GA van, Hondel CAAMJ van de (1982) A new approach for molecular cloning in cyanobacteria: cloning of an Anacystis nidulans met gene using a Tn901-induced mutant. Gene 20:7223–7236
Tandeau de Marsac N, Mazel D, Bryant DA, Houmard J (1985) Molecular cloning and nucleotide sequence of a developmentally regulated gene from the cyanobacterium Calothrix PCC 7601: a gas vacuole protein gene. Nucleic acids Res 20:7223–7236
Thomas PS (1983) Hybridization of denatured RNA transferred of dotted to nitrocellulose paper. Methods Enzymol 100:255–266
Tomioka N, Suguira M (1983) The complete nucleotide sequence of a 16S ribosomal RNA gene from a blue-green alga, Anacystis nidulans. Mol Gen Genet 191:46–50
Touati D (1983) Cloning and mapping of the manganese superoxide dismutase gene (sodA) of Escherichia coli K-12. J Bacteriol 155:1078–1087
Tumer NE, Robinson SJ, Haselkorn R (1983) Different promoters for the Anabaena glutamine synthetase gene during growth using molecular of fixed nitrogen. Nature 306:337–342
Yamakura F (1976) Purification, crystallization and properties of iron-containing superoxide dismutase from Pseudomonas ovalis. Biochim Biophys Acta 422:280–294
Yamakura F (1978) Study on the reconstitution of iron superoxide dismutase from Pseudomonas ovalis. J Biochem (Tokyo) 83:849–857
Author information
Authors and Affiliations
Additional information
Communicated by J. Schell
Rights and permissions
About this article
Cite this article
Laudenbach, D.E., Trick, C.G. & Straus, N.A. Cloning and characterization of an Anacystic nidulans R2 superoxide dismutase gene. Mol Gen Genet 216, 455–461 (1989). https://doi.org/10.1007/BF00334390
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00334390