Summary
A mutant of Escherichia coli was isolated that grew at a normal rate in minimal medium at 26°C, grew at a normal rate in minimal medium at 37°C only if exogenous histidine was supplied, and grew more slowly than normal at 42°C even in the presence of histidine. In very rich media the growth rate of the mutant was normal at 26°C and 30°C, but not at 37°C or 42°C. It may be described as a temperature-conditional histidine bradytroph with a decreased ceiling to its growth rate.
The histidyl-tRNA synthetase of the mutant was found to be abnormal; in crude extracts the enzyme activity was less stable and had approximately a tenfold higher apparent K Mfor histidine than normal.
Under many growth conditions the histidine biosynthetic enzymes in the mutant were derepressed several hundred fold compared to the wild strain, even in the presence of exogenous genous histidine. In general, the degree of derepression in the mutant was proportional to the difference in growth rate between the mutant and normal strains; this relationship, however, did not hold below 30°C or above 37°C.
The properties of the mutant could be related to the properties of its histidyl-tRNA synthetase by assuming that the enzyme participates both in protein synthesis and in histidine biosynthetic enzyme regulation and that at low temperature it functions relatively more effectively in protein synthesis than in repression, while at high temperature it functions relatively more effectively in repression.
Similar content being viewed by others
Abbreviations
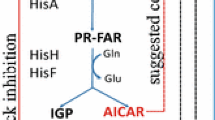
- tRNA:
-
transfer RNA
- AICAR:
-
aminoimidazole carboxamide ribose-5-phosphate
References
Demerec, M., E. A. Adelberg, A. J. Clark, and P. E. Hartman: A proposal for uniform nomenclature in bacterial genetics. Genetics 54, 61 (1966).
Eidlic, L., and F. C. Neidhardt: Role of valyl-sRNA synthetase in enzyme repression. Proc. nat. Acad. Sci. (Wash.) 53, 539 (1965).
Fangman, W. L.: A mutationally altered aminoacyl ribonucleic acid synthetase in Escherchia coli. Ph. D. Thesis, Purdue University (1965).
Fangman, W. L., G. Nass, and F. C. Neidhardt: Immunological and chemical studies of phenylalanyl sRNA synthetase from E. coli. J. mole. Biol. 13, 202 (1965).
Ferroluzzi-Ames, G.: Uptake of amino acids by Salmonella typhimurium. Arch. Biochem. 104, 1 (1964).
Fraenkel, D., and F. C. Neidhardt: Use of chloramphenicol to study control of RNA synthesis in bacteria. Biochim biophys. Acta (Amst.) 53, 96 (1961).
Freundlich, M., R. O. Burns, and H. E. Umbarger: Control of isoleucine, valine and leucine biosynthesis. I. Multivalent repression. Proc. nat. Acad Sci. (Wash.) 48, 1804 (1962).
Gorini, L., and H. Kaufman: Selecting bacterial mutants by the penicillin method. Science 131, 604 (1960).
Lowrey, O. H., N. J. Rosegrough, A. L. Farr, and R. J. Randall: Protein measurement with the phenol reagent. J. biol. Chem. 193, 265 (1951).
Moyed, H. S.: Inter mediates in histidine synthesis: the formation of imidazole glycerolphosphate. In: Methods of enzymology, ed. by S. P. Colowick and N. A. Kaplan, vol. VI, p. 577. New York: Academic Press 1963.
—, and B. Magasanik: The mammalian metabolism of L-histidine. J. biol. Chem. 235, 149 (1959).
Nass, G. and F. C. Neidhardt: Histidyl sRNA synthetase and enzyme repression in Escherichia coli. Bact. Proc. 87, (1966).
Nass, G. and F. C. Neidhardt: Regulation of formation of aminoacyl-ribonucleic acid synthetases in Escherichia coli. Biochim. biophys. Acta (Amst.) 134 (1967).
Ravel, J. M., R. E. Eakin, and W. Shive: Glycine, a precusor of 5(4)-amino-4(5)-imidazole carboxamide. J. biol. Chem. 172, 67 (1948).
Roth, J. R.: Regulatory mutants of Salmonella typhinurium selected by resistence to a histidine analogue, triazolealanine. Fed. Proc. 24, 416 (1965).
—, and B. Ames: Histidine regulatory mutants in Salmonella typhimurium: Histidine regulatory mutants having altered histidyl-tRNA synthetase. J. molec. Biol. 22, 325 (1966).
—, D. N. Anton, and P. E. Hartman: Histidine regulatory mutants in Salmonella typhimurium: Isolation and general properties. J. molec. Biol. 22, 305 (1966).
Schlesinger, S., and B. Magasanik: Effect of α-inethylhistidine on the control of histidine synthesis. J. molec. Biol. 9, 670 (1964).
Silbert, D. F., G. R. Fink, and B. N. Ames: Histidine regulatory mutants in Salmonella typhimurium: A class of regulatory mutants deficient in tRNA for histidine. J. molec. Biol. 22, 335 (1966).
Umbarger, H. E., and B. Brown: Isoleucine and valine metabolism in Escherichia coli. J. biol. Chem. 233, 415 (1958).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Nass, G. Regulation of histidine biosynthetic enzymes in a mutant of Escherichia coli with an altered histidyl-tRNA synthetase. Molec. Gen. Genetics 100, 216–224 (1967). https://doi.org/10.1007/BF00333608
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00333608