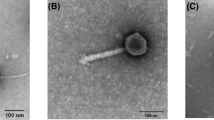
Summary
Clear-plaque phage ϑc, attacking bacitracin-producing strains of B. licheniformis, yields spontaneous temperate mutants at high frequency; the temperate mutants fall into several classes phenotypically different in plaque morphology and properties of lysogenised bacteria. The most common phenotype ϑ3 has DNA restriction fragment patterns identical with those of the parent ϑc; some less common temperate forms, i.e. ϑ1 and ϑ2, produce different restriction fragment patterns, sugesting that a part of the original ϑc DNA has been reorganized or replaced by some foreign genetic material. The changed fragment pattern remains stable upon subsequent passaging of the phage or of the lysogenic bacteria. Neither class of temperate phage mutants gives clearplaque revertants at measurable frequency. Lysogenisation of bacteria with any class of temperate phage confers immunity to all temperate forms and to ϑc; virulent mutants ϑvir, which plate with 100% efficiency on lysogens for ϑ1 and ϑ2 but not for ϑ3, occur in stocks of ϑc at a frequency of 10−7. The mutation from ϑc to ϑvir is not accompanied by any change of the restriction fragment patterns of DNA.
Similar content being viewed by others
References
Adams, M.H.: Bacteriophages, p. 446, New York: Interscience Publishers Inc. 1959
Bott, K., Strauss, B.: The carrier state of Bacillus subtilis infected with the transducing bacteriophage SP 10. Virology 25, 212–225 (1965)
Brenner, S., Streisinger, G., Homes, R.W., Champe, S.P., Barnett, L., Benzer, S., Rees, M.W.: Structural components of bacteriophage. J. molec. Biol. 1, 281–292 (1959)
Cooper, S., Zinder, N.D.: The growth of an RNA bacteriophage: the role of DNA synthesis. Virology 18, 405–411 (1962)
Davis, R.W., Simon, M., Davidson, N.: Electron microscope heteroduplex methods for mapping regions of base sequence homology in nucleic acids. Methods in enzymology, ed. L. Grossman and K. Moldave, Vol. 21, pp. 413–428, 1971
Davis, R.W., Simon, M., Davidson, N.: Electron-microscopic visualization of deletion mutations. Proc. nat. Acad. Sci. (Wash.) 60, 243–250 (1968)
Freese, E., Bautz, E., Bautz-Freese, E.: The chemical and mutagenic specificity of hydroxylamine. Proc. nat. Acad. Sci. (Wash.) 47, 845–855 (1961)
Greene, P.J., Betlach, M.C., Goodman, H.M., Boyer, H.W.: The EcoRI restriction endonuclease. In: Methods in molecular biology (R. Weckner, ed.), Vol. 7, pp. 87–111. New York: Academic Press, 1974
Hemphill, H.E., Whiteley, H.R.: Bacteriophages of Bacillus subtilis. Bact. Rev. 39, 257–315, (1975)
Ito, J., Meinke, W., Hathway, G., Spizizen, J.: Studies on Bacillus subtilis bacteriophage ϕ 15. Virology 56, 110–122 (1969)
Jones, B.R., Reznikoff, W.S.: Isolation of specialized transducing bacteriophages carrying deletions of the regulatory region of the E. coli K12 tryptophan operon. J. Bact. 128, 283–289 (1976)
Ludvik, J., Erbenová, L., Lipavská, H.: Ultrastructure of Bacillus licheniformis bacteriophage BLE and its DNA. Virology 77, 872–875 (1977)
Sonnenshein, A.V., Roscoe, D.H.: The course of phage øe infection in sporulating cells of B. subtilis strain 3610. Virology 39, 265–276 (1969)
Spizizen, J.: Transformation of biochemically deficient strains of Bacillus subtilis by deoxyribonucleate. Proc. nat. Acad. Sci. (Wash.) 44, 1072–1078 (1958)
The, G. de, O'Conner, T.E.: Structure of a murine leukemia virus after disruption with tween-ether and comparison with two myxoviruses. Virology 28, 713–728 (1966)
Thomas, M., Davis, R.W.: Studies on the cleavage of bacteriophage lambda with EcoRI restriction endonuclease. J. molec. Biol. 91, 315–328 (1975)
Wilson, G.H., Williams, M.T., Baney, H.W., Young, F.E.: Characterization of temperate bacteriophage of Bacillus subtilis by the restriction endonuclease EcoRI: evidence for three different temperate bacteriophages. J. Virol. 14, 1013–1016 (1974)
Wilson, G.H., Young, F.E.: Isolation of a sequence-specific endonuclease BamHI from Bacillus amyloliquefaciens H. J. molec. Biol. 97, 123–125 (1975)
Yamamoto, K.R., Alberts, B.M., Benzinger, R., Lawthorne, L., Treiber, G.: Rapid bacteriophage sedimentation in the presence of polyethylen glycol and its application to large-scale virus purification. Virology 40, 734–744 (1970)
Author information
Authors and Affiliations
Additional information
Communicated by W. Arber
Rights and permissions
About this article
Cite this article
Doskočil, J., Forstová, J. & Štokrová, J. Temperate and virulent forms of phage theta attacking Bacillus licheniformis . Molec. Gen. Genet. 160, 311–317 (1978). https://doi.org/10.1007/BF00332974
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00332974